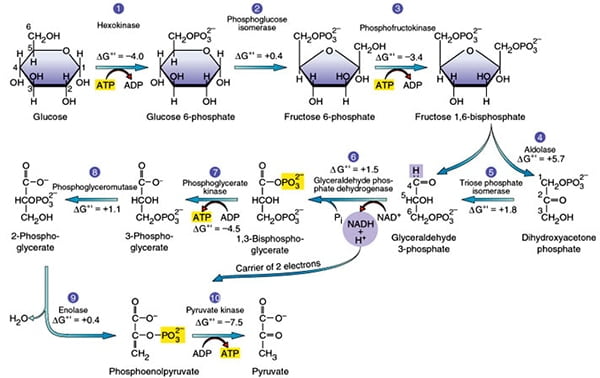
Glycolysis is the metabolic process that serves as the foundation for both aerobic and anaerobic cellular respiration. In glycolysis, glucose is converted into pyruvate. Glucose is a six- memebered ring molecule found in the blood and is usually a result of the breakdown of carbohydrates into sugars. It enters cells through specific transporter proteins that move it from outside the cell into the cell’s cytosol. All of the glycolytic enzymes are found in the cytosol.
The overall reaction of glycolysis which occurs in the cytoplasm is represented simply as:
C6H12O6 + 2 NAD+ + 2 ADP + 2 P —–> 2 pyruvic acid, (CH3(C=O)COOH + 2 ATP + 2 NADH + 2 H+
Step 1: Hexokinase
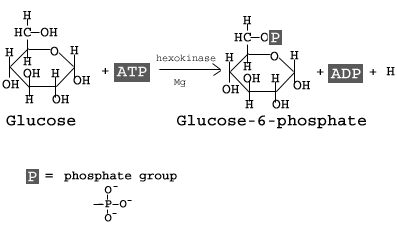
The first step in glycolysis is the conversion of D-glucose into glucose-6-phosphate. The enzyme that catalyzes this reaction is hexokinase.
Details:
Here, the glucose ring is phosphorylated. Phosphorylation is the process of adding a phosphate group to a molecule derived from ATP. As a result, at this point in glycolysis, 1 molecule of ATP has been consumed.
The reaction occurs with the help of the enzyme hexokinase, an enzyme that catalyzes the phosphorylation of many six-membered glucose-like ring structures. Atomic magnesium (Mg) is also involved to help shield the negative charges from the phosphate groups on the ATP molecule. The result of this phosphorylation is a molecule called glucose-6-phosphate (G6P), thusly called because the 6′ carbon of the glucose acquires the phosphate group.
Step 2: Phosphoglucose Isomerase
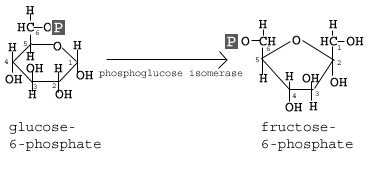
The second reaction of glycolysis is the rearrangement of glucose 6-phosphate (G6P) into fructose 6-phosphate (F6P) by glucose phosphate isomerase (Phosphoglucose Isomerase).
Details:
The second step of glycolysis involves the conversion of glucose-6-phosphate to fructose-6-phosphate (F6P). This reaction occurs with the help of the enzyme phosphoglucose isomerase (PI). As the name of the enzyme suggests, this reaction involves an isomerization reaction.
The reaction involves the rearrangement of the carbon-oxygen bond to transform the six-membered ring into a five-membered ring. To rearrangement takes place when the six-membered ring opens and then closes in such a way that the first carbon becomes now external to the ring.
Step 3: Phosphofructokinase
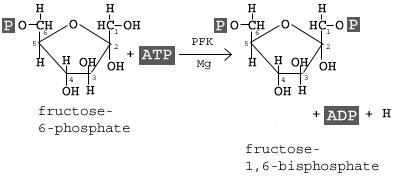
Phosphofructokinase, with magnesium as a cofactor, changes fructose 6-phosphate into fructose 1,6-bisphosphate.
Details:
In the third step of glycolysis, fructose-6-phosphate is converted to fructose- 1,6-bisphosphate (FBP). Similar to the reaction that occurs in step 1 of glycolysis, a second molecule of ATP provides the phosphate group that is added on to the F6P molecule.
The enzyme that catalyzes this reaction is phosphofructokinase (PFK). As in step 1, a magnesium atom is involved to help shield negative charges.
Step 4: Aldolase
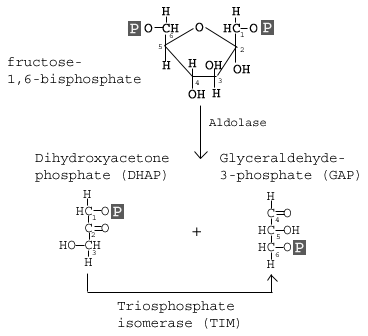
The enzyme Aldolase splits fructose 1, 6-bisphosphate into two sugars that are isomers of each other. These two sugars are dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (GAP).
Details:
This step utilizes the enzyme aldolase, which catalyzes the cleavage of FBP to yield two 3-carbon molecules. One of these molecules is called glyceraldehyde-3-phosphate (GAP) and the other is called dihydroxyacetone phosphate (DHAP).
Step 5: Triosephosphate isomerase
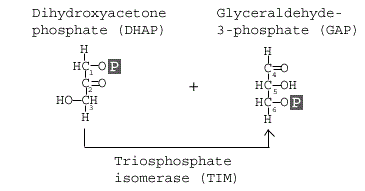
The enzyme triosephosphate isomerase rapidly inter- converts the molecules dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (GAP). Glyceraldehyde phosphate is removed / used in next step of Glycolysis.
Details:
GAP is the only molecule that continues in the glycolytic pathway. As a result, all of the DHAP molecules produced are further acted on by the enzyme Triosephosphate isomerase (TIM), which reorganizes the DHAP into GAP so it can continue in glycolysis. At this point in the glycolytic pathway, we have two 3-carbon molecules, but have not yet fully converted glucose into pyruvate.
Step 6: Glyceraldehyde-3-phosphate Dehydrogenase
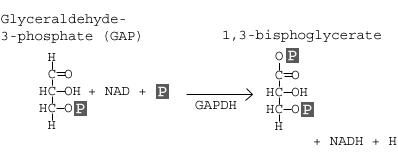
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) dehydrogenates and adds an inorganic phosphate to glyceraldehyde 3-phosphate, producing 1,3-bisphosphoglycerate.
Details:
In this step, two main events take place: 1) glyceraldehyde-3-phosphate is oxidized by the coenzyme nicotinamide adenine dinucleotide (NAD); 2) the molecule is phosphorylated by the addition of a free phosphate group. The enzyme that catalyzes this reaction is glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
The enzyme GAPDH contains appropriate structures and holds the molecule in a conformation such that it allows the NAD molecule to pull a hydrogen off the GAP, converting the NAD to NADH. The phosphate group then attacks the GAP molecule and releases it from the enzyme to yield 1,3 bisphoglycerate, NADH, and a hydrogen atom.
Step 7: Phosphoglycerate Kinase
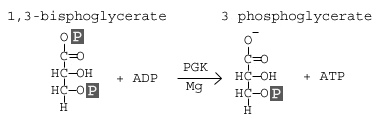
Phosphoglycerate kinase transfers a phosphate group from 1,3-bisphosphoglycerate to ADP to form ATP and 3-phosphoglycerate.
Details:
In this step, 1,3 bisphoglycerate is converted to 3-phosphoglycerate by the enzyme phosphoglycerate kinase (PGK). This reaction involves the loss of a phosphate group from the starting material. The phosphate is transferred to a molecule of ADP that yields our first molecule of ATP. Since we actually have two molecules of 1,3 bisphoglycerate (because there were two 3-carbon products from stage 1 of glycolysis), we actually synthesize two molecules of ATP at this step. With this synthesis of ATP, we have cancelled the first two molecules of ATP that we used, leaving us with a net of 0 ATP molecules up to this stage of glycolysis.
Again, we see that an atom of magnesium is involved to shield the negative charges on the phosphate groups of the ATP molecule.
Step 8: Phosphoglycerate Mutase
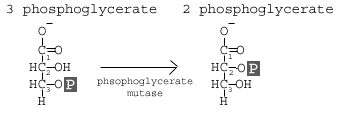
The enzyme phosphoglycero mutase relocates the P from 3- phosphoglycerate from the 3rd carbon to the 2nd carbon to form 2-phosphoglycerate.
Details:
This step involves a simple rearrangement of the position of the phosphate group on the 3 phosphoglycerate molecule, making it 2 phosphoglycerate. The molecule responsible for catalyzing this reaction is called phosphoglycerate mutase (PGM). A mutase is an enzyme that catalyzes the transfer of a functional group from one position on a molecule to another.
The reaction mechanism proceeds by first adding an additional phosphate group to the 2′ position of the 3 phosphoglycerate. The enzyme then removes the phosphate from the 3′ position leaving just the 2′ phosphate, and thus yielding 2 phsophoglycerate. In this way, the enzyme is also restored to its original, phosphorylated state.
Step 9: Enolase
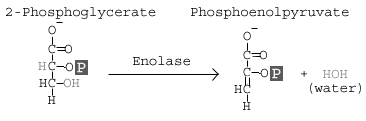
The enzyme enolase removes a molecule of water from 2-phosphoglycerate to form phosphoenolpyruvic acid (PEP).
Details:
This step involves the conversion of 2 phosphoglycerate to phosphoenolpyruvate (PEP). The reaction is catalyzed by the enzyme enolase. Enolase works by removing a water group, or dehydrating the 2 phosphoglycerate. The specificity of the enzyme pocket allows for the reaction to occur through a series of steps too complicated to cover here.
Step 10: Pyruvate Kinase
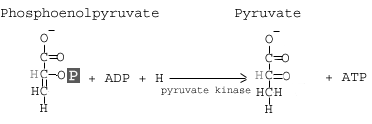
The enzyme pyruvate kinase transfers a P from phosphoenolpyruvate (PEP) to ADP to form pyruvic acid and ATP Result in step 10.
Details:
The final step of glycolysis converts phosphoenolpyruvate into pyruvate with the help of the enzyme pyruvate kinase. As the enzyme’s name suggests, this reaction involves the transfer of a phosphate group. The phosphate group attached to the 2′ carbon of the PEP is transferred to a molecule of ADP, yielding ATP. Again, since there are two molecules of PEP, here we actually generate 2 ATP molecules.
Steps 1 and 3 = – 2ATP
Steps 7 and 10 = + 4 ATP
Net “visible” ATP produced = 2.
Immediately upon finishing glycolysis, the cell must continue respiration in either an aerobic or anaerobic direction; this choice is made based on the circumstances of the particular cell. A cell that can perform aerobic respiration and which finds itself in the presence of oxygen will continue on to the aerobic citric acid cycle in the mitochondria. If a cell able to perform aerobic respiration is in a situation where there is no oxygen (such as muscles under extreme exertion), it will move into a type of anaerobic respiration called homolactic fermentation. Some cells such as yeast are unable to carry out aerobic respiration and will automatically move into a type of anaerobic respiration called alcoholic fermentation.

Thanks for describing this in detail. I am wondering though, glycolysis described here may thought to be for human metabolism, but I am not sure if this is the only pathway for glycolysis in humans. I am a believer in exogenous enzyme helping human (and mammalian) metabolism, by means of acquiring it gastronomically. Modern human food does not include metabolic enzymes in general. but it does not mean it is not possible for exogenous enzyme to work in the body, nor it is unhealthy to acquire one.
Are those enzymes you described the only enzymes coded in human DNA?
Thanks for the explanation sir.
It was a very good one.
I tried to understand it during my biochemistry class but was difficult, but with this, it’s going to be easy for me to understand
please someone tell me which are phosphorylated steps and splitting steps ? indicating by step number.
step 7 and 10 produce atp thru subtrate level phosphyrlation
Is pyruvate produced through a process of glycolysis?
Yes it’s produce at the end in an aerobic condition
How many ATP produced when fructose or glucose enters the glycolysis pathway
Number of total atp produced is 2
a net of two ATPs is produced
Net ATPs produced are 2 but 2 NADH are also produced which in Electron Transport Chain each produces 3 ATPs so according to this idea 3×2=6 ATPs and overall ATPs produced are 2+6=8 ATPs.
2 ATP
2 ATP molecules are produced for every molecules of glucose that goes into glycolysis
4, ATP but, again 2atp were used in the process so our net gain is:2 ATP
overall 4 ATP molecules are produced, but since 2 molecules of ATP are used in steps 1 and 3, a net of 2 molecules are produced
10 steps in glycolysis, how many of them consume ATP?
ATP Consumed in step one and three respectively
2 steps consume ATP ie the phosphorylation step that occurs at first, the other phosphorylation step that occurs after isomerism
what happens to the hydrogens in step 1 and step 3 ?
This is for everyone asking about this.
Usually all the remaining products can be used for a new source a energy.
Remember
Energy can niether be created nor distroyed
Study the mechanism critically
They are only being converted to a different form
Actually they have been converted to other forms, I could not understand the whole process but the char has made it simplest
Forms water or is used up for other source of energy
They are converted to water
Your question: “what happens to the hydrogens in step 1 and step 3 ?”
1) In reaction 1, glucose gets phosphorylated (i.e addition of phosphate group) at its 6th carbon atom (i.e the CH2OH molecule), so, the bond formation between the phosphate group and the CH2OH leads to the ‘removal of the H atom of the OH (hydroxyl) group’ from the CH2OH molecule. The 6th carbon atom of glucose looks like this ‘C+H+H+OH’ i.e C has H+H+OH attached to it. So, phosphate comes in, removes the H of the OH, and attaches to the remaining ‘O’ of the OH. That’s why reaction 1 has Glucose-6-Phosphate + ADP + ‘H’.
2) For reaction 3, it’s the same process (as reaction 1) by which another phosphate group is added to fructose-6-phosphate, but this time on its 1st carbon atom, phosphate goes in and removes the H of the OH on the CH2OH on carbon 1 of fructose, attaches itself to carbon 1, thus release another H atom.
Although the ADP is the result of hydrolysis of ATP i.e when you remove just one phosphate group from ATP (Adenosine Triphosphate: Adenosine with three phosphate groups), you get ADP (Adenosine Diphosphate: Adenosine with two phosphate groups).
lost 1 H+ for each step
Glucose is phosphorylated in the first step because you want the glucose to be trapped in the cell to continue in the pathway. Without this, the glucose will be sent out of the cell via transporters. This also initiates the reduction of instability of the glucose to increase its reactivity.
“This also initiates the reduction of instability of the glucose to increase its reactivity.”
I thought the point was to increase the instability of glucose, by adding the second phosphate to the other side to make fructose 1, 6 bisphosphate. Now the two phosphates push away from each other and make it easy work for aldolase to split the molecule in two.
Thanks a lots. But how do we do the calculation under this?
Thanks but I have a question that why glucose is phosphorylated in the first step of glycolysis
to realise one Phosphate from ATP, then ATP(adenosin triphosphate) turns into ADP(adenosin diphosphate), the phospate realised will be added to glucose, then glucose turns into glucose 6 Phosphate.
To capture glucose in the cell first it phosphorylated then the next process is proceed
Well, this occurs due to the fact that phosphorylation can keep a molecule from diffusing out of its position; making it more reactive by borrowing a phosphate molecule from ATP.
Adenosine Triphosphate (3 phosphate)
Which is converted to ADP (Adenosine Diphosphate)
GLUCOSE-
GLUCOSE 6 PHOSPHATE
Why not other compound used to make glucose become active, but only phosphate group is used?
In the last reaction PEPA +ADP…. from where ADP com from
It gets attached to 6th carbon as it’s easily accessible than other carbons and needs less energy
It also happens to keep the glucose in the cell insted of losing it out
glucose is phosphorylated inorder to trap it in the cell’s cytoplasm. This is because phosphorylated glucose cannot diffuse through the lipid bilayer.
In g3p dehydrogenase. . How oxygen is attached to po3 from where it come.. N how h+ is formed the released H is involved to form and to nadh
Actually G3P is first converted to 1-3- bi phosphogleraldehyde with the help of H3PO4 & H20. It is a non enzymatic reaction. 1-3- bi phosphogleraldehyde is then converted to 1-3 bi phosphogleceric acid. Here NAD is reduced to NADH. But for this conversion 1/2 O2 is required, because NADH must be oxidized to form NAD, ( NAD is electron carrier) without which this conversion will not take place ( enzyme required is G-3 phosphate Dehydrogenase). Therefore 1) for oxidation of Nadh 2) High energy of electron is then produce ATP, and as a terminal acceptor of electron, O2 is required. ( 1/2 O2 x 2 cycle = 1 mol of O2 required). Hope this is clear.
But what is the electron here,is it H- and if it is then how H- is produced???
thanks alot but i have a question, why is it that normally during phosphorylation ATP is consumed but during glyceraldehyde phosphate dehydrogenase when the inorganic phosphate was added to the molecule, ATP was not consumed why??
Because there are free floating inorganic phosphates in the cytosol of the cell that are used for the phosphorylation of that substrate where ATP is not required.
Because dehygenase enzyme requires NAD as a cofactor
this is exactly what my teacher has taught in the classroom.
reading this seems as if i am reading his own written steps of the glycolysis process.
Thank you very much.
This is a great survey of glycolysis. Our class does go in more depth, but I can’t think of a better resource to start with. Thanks so much! I shared it with all my classmates. I hope your page gets sore from all the web hits it is gonna get!
Each NADH will produce approx 2.5 ATP during the election transport chain.
Thanks I appreciate this impressive literature ,quite educative we need more of this concerning glycogen
Please i want to understand how the net yield of 8ATPs in aerobic glycolysis is realized.
Thank you
Thank God I came across this.. Best one I’ve read on Glycolysis so far.. Even better than my textbooks..
Were do the other hydrogens goes after the splitting of fructose in step 4?
when one AtP molecules is produce and other one is consumed so what effect on glycolysis???
It will result to low yield at the end of the process
In the last step where phosphoenol pyruvate is converted to pyruvic acid! Where is the Hydrogen atom coming from?
why is there a H atom again on the 2 CARBON in the 10 step when we removed it as H2O in the 9th step.
Thank you very much, I thought triose phosphate was the main intermediate which continues glycolysis. Thanks fr clearing this dilemma
I’ve searched and searched for the easiest to understand discussion about Glycolysis and I was not disappointed by the way you presented the complexity of the process. (Honestly, I can feel how you really want to let the readers understand the concepts in a friendly language to help them keep up with every step.) Thanks a lot!
Honestly this is amazing. l couldn’t get it right in class ,but I understand it now. Thanks alot
Dear sir the chemical formula of phosphoenole pyruvate is c3h5o6p and you write c3h4o3p.
Pls help me and explain how fruit you just taken will enter into glycolytic pathway.
most of the fruits you eat will breakdown as simpler substances so that cell can consume it. in further processes glucose will be converted to pyruvate.In simple words most of the food items contains glucose or its combined forms like sucrose .etc.when you eat an apple you are taking in glucose .glycolytic cycle occurs in cell organelles like mitochondria .etc.these all happens the very moment when something reaches your stomach.
dear thanks for making it easy. pls can you simplify electron transport chain/ oxidative phosphorylation.
I don’t actually knows how to express to you my thanks, because the way you splits, disintegrates, and break down this glycolic pathway I’m so impressed. To be honest before I found this, I suffered a lot on how to understand this thing.Because I didn’t attends the lectures at school when the lecturer is conducting it. But now I’m fully convinced and fully understand it. Thank You so much.
{I wish that, all your wishes be granted}
Do you have the same excelently written Krebs Cycle as you have done in the glycolisis cycle ?. You have done an outstanding job.
Well written notes.Please mark on structure how the six carbon breaks into 3carbons compounds in step 4
For anyone who is still confused on step 6 watch https://www.youtube.com/watch?v=_sXCPL241Hk 0:00 – 4:47
Kindly give an explanation behind the negative 2 ATP molecules in the first and third steps
Kindly describe the process in the mitochondria as well. Also please do the same for gluconeogenesis, glycogenesis and glucogenolysis.
I like the way all the steps have been outlined for easy understanding. I found this very helpful. With some level of effort, I now have all the 10 steps on my finger tips for my biochemistry class. Thank you so much for sharing.
I have a doubt,from where does the phosphate in step 6 come from?
It just says that phosphate is added.
Anyway the answer was useful,Thank you!
it would have been much better if the energy released or absorbed at each stage was included.
I don’t understand the role of magnesium, can you explain it more clearly?
I agree
The role is clear that it shields the highly reactive negative charge phosphate from reacting with ADP molecules but how does the cofactor and enzyme distinguish between ADP and ATP when both have a difference of one phosphate group.
Unexplained: H2O removal from Phosphoglycerate should leave PEP and Pyruvate with 2 H. But Pyruvate has 4 H. Does 2H reenter PEP
Good discussion , I was enlightened by the specifics , Does anyone know if I could acquire a blank WI WB-42 example to use ?
For each molecule of glucose two molecules of payruvic acid (payruvate) formed…
Am happy to get this note
thanks sir
Ghana Kumasi polytechnic
please I want to know this
since there were two molecules of PEP,was two molecules of pyruvate compound formed?
yeah, two molecules of pyruvate are formed and they all jump into citric acid cycle(kreb cycle) with the help of pyruvate dehydrogenase.
now this is the best thing i have read so far on glycolysis.even my text book can’t explain it well.thank you very much.
This is easier than the ones I’ve encountered and makes my work a lot more easy I need this for my write up and I need to explain it as well for my final year paper as an Animal Nutritionist thanks a lot DR
Archea can srvive in harsh environmenatl conditions because of its specific Cell wall and cell membrane compositions.
Thanx for the illustration. I have a query regarding structure of glucose. You have placed hydroxyl group in structure of glucose down in first carbon. Same is the case in second carbon, but you have placed hydroxyl group in third carbon up. Does it have to be so specific? I mean, cant we place hydroxyl group in first carbon up or hydroxyl group in third carbon down? I have save same question regarding placement of hydroxyl group in 3 carbon structures ie left or right.
In aerobic glucose metabolism, the oxidation of citric acid uses ADP and Mg²+, which will increase the speed of reaction: Iso-citric acid + NADP (NAD) — isocitrate dehydrogenase (IDH) = alpha-ketoglutaric acid.
In the Krebs cycle (the citric cycle), IDH1 and IDH2 are NADP+-dependent enzymes that normally catalyze the inter-conversion of D-isocitrate and alpha-ketoglutarate (α-KG). The DH1 and IDH2 genes are mutated in > 75% of different malignant diseases. Two distinct alterations are caused by tumor-derived mutations in IDH1 or IDH2: loss of its normal catalytic activity during the production of α-ketoglutarate (α-KG) and the gain of catalytic activity to produce 2-hydroxygulatrate (2-HG).
This product is a competitive inhibitor of multiple α-KG-dependent dioxygenases, including histone, demethylases, prolyl-4-hydroxylase and the TET enzymes family (Ten-Eleven Translocation-2), resulting in genome-wide alternations in histones and DNA methylation.
IDH1 and IDH2 mutations have been observed in myeloid malignancies, including de novo and secondary AML (15%–30%), and pre-leukemic clone malignancies, including myelodysplastic syndrome and myeloproliferative neoplasms (85% of the chronic phase and 20% of transformed cases in acute leukemia).
The energetic sum of anaerobic glycolysis is ΔGo = -34.64 kcal/mol. However, a glucose molecule contains 686 kcal/mol and the energy difference (654.51 kcal) remains a potential for un-controlled reactions in carcinogenesis. The transfer of electrons from NADPH in each place of the conserved unit of energy transmits conformational exchanges of mitochondrial ATPases. In the reaction, ADP³+ P²¯ + H²– ATP + H2O it is a reversible reaction. The terminal oxygen from ADP binds the atom P2¯ by forming an intermediate pentacovalent length and synthesizing the molecular complexes ATP and H2O. This reaction requires Mg²+ and ATP-synthetase, which is known as the H+-ATPase or the Fo-F1-ATPase complex, where FO is a conductor proton and F1 is synthesized.
Mg2+ stabilizes the mitochondrial membrane via the high electronegativity of its electrons. In contrast, intracellular calcium induces mitochondrial swelling and aging. Mg2+ generally interacts with substrates via the inner coordination sphere, stabilizing anions or reactive intermediates, binding ATP and activating the molecule for nucleophilic attack.
Dr. Aurel
I’m glad to see this post, as a student of nutrition for better health and aging. How does this relate to Diabetes? Can you connect the dot for the general public? I’ve read dozens of books on diet and nutrition, but only getting confused, without conceptual biological-chemical bases to validate many contradictory claims.
I am glad to see that you included the delta-G values in the principal figure. These are very important for helping students appreciate how the flow operates in these pathways, but the values are often left out of figures for the sake of simplicity. At the same time, I would recommend adding arrows for the reverse reactions, perhaps with length indicating the free energy vector, to further emphasize and distinguish the freely reversible from essentially irreversible reactions. It might also help to add both the free energy values and the reverse arrows to the single-step figures, as well. Overall, this is a pretty good study review.
Nice theory