Motility is the ability of an organism to move by itself by means of propeller-like flagella unique to bacteria or by special fibrils that produce a gliding form of motility. Motile bacteria move using flagella, thread like locomotor appendages extending outward from the plasma membrane and cell wall either single flagellum or multiple flagella. Motility has long been recognized as an important taxonomic tool and biological characteristic of microorganisms. The presence of flagella occurs primarily in bacilli but there are a few flagellated cocci, thus motility is a very important means of identification in the family Enterobacteriaceae. From the early days in the field of microbiology, the ability of bacteria to move has been used as a means of differentiation and classification of organisms.
Objective
- To determine the motility of bacterium.
- To differentiate between motile and non motile bacteria.
Principle
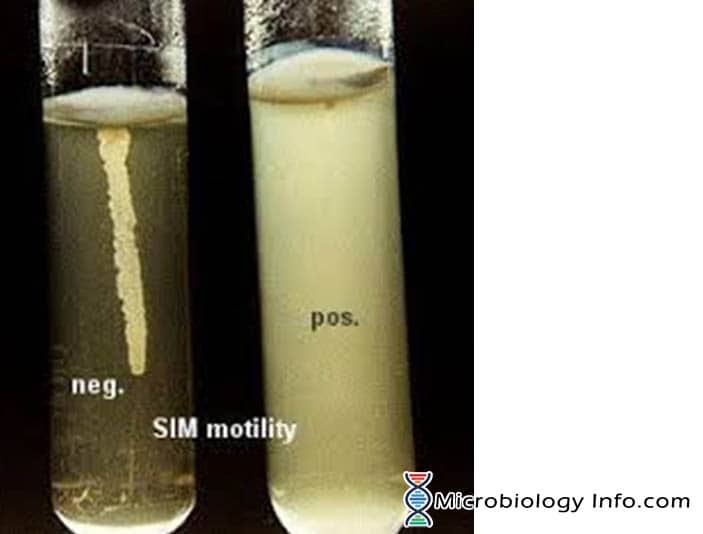
Motility by bacterium is mostly demonstrated in a semi solid agar medium. In semi-solid agar media, motile bacteria ‘swarm’ and give a diffuse spreading growth that is easily recognized by the naked eye.The medium mainly used for this purpose is SIM medium (Sulphide Indole Motility medium) which is a combination differential medium that tests three different parameters, Sulfur Reduction, Indole Production and Motility. This media has a very soft consistency that allows motile bacteria to migrate readily through them causing cloudiness. The inoculum is stabbed into the center of a semisolid agar deep. Bacterial motility is evident by a diffuse zone of growth extending out from the line of inoculation. Some organisms grow throughout the entire medium, whereas others show small areas or nodules that grow out from the line of inoculation. The non-motile bacteria will only grow in the soft agar tube and only the area where they are inoculated.
Media:
SIM Medium
Pancreatic digest of casein 20.0g, Peptic digest of animal tissue 6.1g, Agar 3.5g, Fe(NH4)2(SO4)2·6H2O 0.2g, Na2S2O3·5H2O 0.2g, pH 7.3 ± 0.2 at 25°C
Method
- Touch a straight needle to a colony of a young (18- to 24-hour) culture growing on agar medium.
- Stab once to a depth of only 1/3 to ½ inch in the middle of the tube. Be sure to keep the needle in the same line it entered as it is removed from the medium.
- Incubate at 35°-37°C and examine daily for up to 7 days.
- Observe for a diffuse zone of growth flaring out from the line of inoculation.
Expected Results
- Positive: Diffuse, hazy growths that spread throughout the medium rendering it slightly opaque.
- Negative: Growth that is confined to the stab-line, with sharply defined margins and leaving the surrounding medium clearly transparent.
Uses
- It is used for the differentiation of microorganisms on the basis of motility in a laboratory setting.
- It is performed to assign taxonomic classification to organisms.
- Motility tests are important in characterization of pathogens.
- The tests are often employed in identification protocols in the family Enterobacteriaceae
- Motility test is also used for the species differentiation of gram positive cocci, Enterococci. Enterococcus faecium and E. faecalis are non-motile, whereas E. gallinarum and E. casseliflavus/E. flavescens generally are motile.
Limitations
- Some organisms will not display sufficient growth in this medium to make an accurate determination, and additional follow-up testing is required.
- It is recommended that biochemical, immunological, molecular, or mass spectrometry testing be performed on colonies from pure culture for complete identification.
- False-negative reactions may occur if bacterial flagella are damaged due to heating, shaking, or other trauma. Such environmental shock will render the organism non-motile.
- Organisms which are weakly motile may result in false-negative reactions.
- When inoculating semi-solid media, it is important that the inoculating needle be removed along the exact same line used to inoculate the medium. A fanning motion may result in growth along the stab line that may result in false-positive interpretation.
References
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- Cappuccino J.G. and Sherman N. 2008. Microbiology: A Laboratory Manual, 8th ed. Pearson Benjamin Cummings, San Francisco, CA, USA.
- vlab.amrita.edu/?sub=3&brch=73&sim=697&cnt=1
- www.asmscience.org/content/education/protocol/protocol.3658
- https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/MotilityTestMedia.htm
Similar Posts:
- List of culture media used in microbiology with their uses
- Flagella – Introduction, Types, Examples, Parts, Functions and Flagella Staining- Principle, Procedure and Interpretation
- The Enterotube™ II – Procedure, Result Interpretation, Merits and Limitations
- Simmons Citrate Agar- Composition, Principle, Uses, Preparation and Result Interpretation
