Lowenstein-Jensen (LJ) is the selective medium which is used for the cultivation and isolation of Mycobacterium species. It was developed by Lowenstein who incorporated congo red and malachite green to inhibit unwanted bacteria. The present formulation, a glycerated egg-based medium, is based upon Jensen’s modification. Jensen’s version eliminates congo red and uses a moderate concentration of malachite green to prevent growth of the majority of contaminants surviving decontamination of the specimen. This formulation also encourages the earliest possible growth of mycobacteria.
Composition of LJ Medium
| Ingredients | Amount |
|---|---|
| Potato Flour (Potato Starch) | 30.0 gm |
| L-Asparagine | 3.6 gm |
| Monopotassium Phosphate | 2.4 gm |
| Magnesium Citrate | 0.6 gm |
| Malachite Green | 0.4 gm |
| Magnesium Sulfate | 0.24 gm |
| Glycerol | 12 ml |
| Egg suspension | 1000 ml |
| Distilled Water | 600 ml |
For cultivation of M. bovis, glycerol is omitted and sodium pyruvate is added.
Principle of LJ Medium
L-Asparagine and Potato Flour are sources of nitrogen and vitamins. Monopotassium Phosphate and Magnesium Sulfate enhance organism growth and act as buffers. Malachite green, prevent the growth of the majority of contaminants surviving decontamination of the specimen while encouraging the growth of Mycobacteria. Egg Suspension provide fatty acids and protein required for the metabolism of mycobacteria. When heated, the egg albumin coagulates, thus providing a solid surface for inoculation. Glycerol serves as a carbon source and is favorable to the growth of the human type tubercle bacillus while being unfavorable to the bovine type.
Uses of LJ Medium
- It is used for the diagnosis of Mycobacterial infections.
- It is used for testing antibiotic susceptibility of isolates.
- It is also used for differentiating different species of mycobacterium (by colony morphology, growth rate, biochemical characteristics and microscopy).
Preparation of LJ Medium
- Dissolve 37.3 gm of the medium in 600 ml of distilled water containing 12 ml of glycerol.
- Heat if necessary to dissolve the medium completely.
- Autoclave at 121°C for 15 minutes.
- Prepare 1000 ml of a uniform suspension of fresh eggs under aseptic conditions. Avoid whipping air into suspension during the collection and mixing.
- Aseptically mix the 1000 ml of egg suspension with 600 ml of the sterile Lowenstein-Jensen Medium cooled to 50 – 60°C, avoiding air bubbles.
- Dispense the finished medium into sterile screw-cap test tubes.
- Place the tubes in a slanted position and heat at 85°C for 45 minutes.
Colony Morphology on LJ Medium
Cultures should be read within 5 to 7 days after inoculation and once a week thereafter for up to 8 weeks.
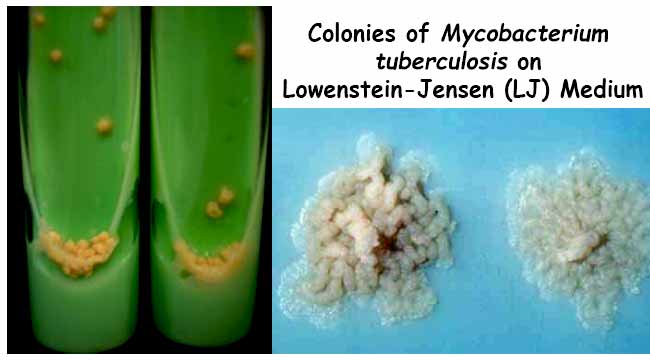
Typical non pigmented, rough, dry colonies are seen on LJ medium. The green color of the medium is due to the presence of malachite green which is one of the selective agents to prevent growth of most other contaminants.
Limitations of LJ Medium
- It is recommended that biochemical and/or serological tests be performed on colonies from pure culture for complete identification.
- LJ Media require incubation in a 5-10% CO2 atmosphere in order to recover mycobacteria. Mycobacteria, for unknown reasons, are not recovered well from candle extinction jars.
- Negative culture results do not rule-out active infection by mycobacteria.
- Due to nutritional variation, some strains may be encountered that grow poorly or fail to grow on this medium. Further tests are necessary for confirmation of Mycobacterium spp.
References:
- Hardy Diagnostics: LOWENSTEIN JENSEN (LJ) MEDIA
- Microbiology In Pictures
- Laboratory Info
- Wong’s Virology
- Medico Tips
- Dynamicro LABS
- E&O Laboratories Ltd
- Sigma-Aldrich Co. LLC: L3910 Lowenstein Jensen medium base
- HiMedia Laboratories: Lowenstein Jensen Medium (L.J. Medium)
- BBL Mycobactosel L-J Medium
- Acumedia Manufacturers, Inc.: LOWENSTEIN – JENSEN MEDIUM (7245)
- Wikipedia

Does pH affect LJ color?
What should be the exact volume of LJ(Solid) media that should be dispensed in screw cap sterile tube?
12-15 ml.
Volume may deffer as per inspissator you have. Volume is standardized in such a way that media should reach upto neck of Mccartney bottle in slanting position on inspissator. Media should not touch cap of bottle. Once volume is standardized you can measure volume.
Please I found it very difficult harvesting H37Ra cell I cultured on LJ slant.Each time I tried to scrape out the cells with a loop ,the green coloured medium followed so much that I could not harvest pure cells.
So I decided to homogenise /sonicate every thing hoping that during lysis only bacteria cells will be lysed and I could get the material of my interest in the supernatant.Is this approach right?Can this affect my result?
The topic is very much informative.
Please show few image of Mycobacteruim bovis culture.
What is the sterility acceptance range for in-house prepared LJ’s?
Mycobacterium Tuberculosis grows very slowly then how it is creating such disaster in human world?
how do one isolate virus from a medium without killing or rupturing the morphology of the organism