In 1922, Dorner published a method for staining endospores. Shaeffer and Fulton modified Dorner’s method in 1933 to make the process faster The endospore stain is a differential stain which selectively stains bacterial endospores. The main purpose of endospore staining is to differentiate bacterial spores from other vegetative cells and to differentiate spore formers from non-spore formers.
Principle of Endospore Staining
Bacterial endospores are metabolically inactive, highly resistant structures produced by some bacteria as a defensive strategy against unfavorable environmental conditions. The bacteria can remain in this suspended state until conditions become favorable and they can germinate and return to their vegetative state.
In the Schaeffer-Fulton`s method, a primary stain-malachite green is forced into the spore by steaming the bacterial emulsion. Malachite green is water soluble and has a low affinity for cellular material, so vegetative cells may be decolorized with water. Safranin is then applied to counterstain any cells which have been decolorized. At the end of the staining process, vegetative cells will be pink, and endospores will be dark green.
Spores may be located in the middle of the cell, at the end of the cell, or between the end and middle of the cell. Spore shape may also be of diagnostic use. Spores may be spherical or elliptical.
Reagents used for Endospore Staining
Primary Stain: Malachite green (0.5% (wt/vol) aqueous solution)
0.5 gm of malachite green
100 ml of distilled water
Decolorizing agent
Tap water or Distilled Water
Counter Stain: Safranin
Stock solution (2.5% (wt/vol) alcoholic solution)
2.5 gm of safranin O
100 ml of 95% ethanol
Procedure of Endospore Staining
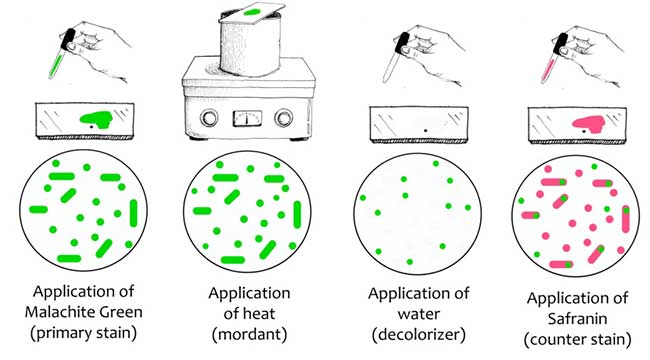
- Take a clean grease free slide and make smear using sterile technique.
- Air dry and heat fix the organism on a glass slide and cover with a square of blotting paper or toweling cut to fit the slide.
- Saturate the blotting paper with malachite green stain solution and steam for 5 minutes, keeping the paper moist and adding more dye as required. Alternatively, the slide may be steamed over a container of boiling water.
- Wash the slide in tap water.
- Counterstain with 0.5% safranin for 30 seconds. Wash with tap water; blot dry.
- Examine the slide under microscope for the presence of endospores. Endospores are bright green and vegetative cells are brownish red to pink.
Result of Endospore Staining
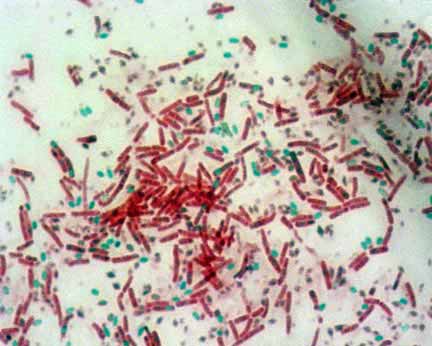
Endospores: Endospores are bright green.
Vegetative Cells: Vegetative cells are brownish red to pink.
Spores may be located in the middle of the cell, at the end of the cell, or between the end and middle of the cell. Spore shape may also be of diagnostic use. Spores may be spherical or elliptical.
Endospore Staining by Dorner’s Method
Carbolfuchsin stain
0.3 gm of basic fuchsin
10 ml of ethanol, 95% (vol/vol)
5 ml of phenol, heat-melted crystals
95 ml of distilled water
Dissolve the basic fuchsin in the ethanol; then add the phenol dissolved in the water.
Mix and let stand for several days. Filter before use.
Decolorizing solvent (acid-alcohol)
97 ml of ethanol, 95% (vol/vol)
3 ml of hydrochloric acid (concentrated)
Counterstain (Nigrosin solution)
10 gm of nigrosin
100 ml of distilled water
Procedure
- Take a clean grease free slide and make smear using sterile technique.
- Air dry and heat fix the organism on a glass slide and cover with a square of blotting paper or toweling cut to fit the slide.
- Saturate the blotting paper with carbolfuchsin and steam for 5 to 10 minutes, keeping the paper moist and adding more dye as required. Alternatively, the slides may be steamed over a container of boiling water.
- Remove the blotting paper and decolorize the film with acid-alcohol for 1 minute; rinse with tap water and blot dry.
- Further take a drop of nigrosine on one end of a slide and make a thin film of a stain all over the smear with the help of other slide.
- Allow the film of Nigrosin to air dry.
- After air drying observe the slide under oil immersion.
Vegetative cells are colorless, endospores are red, and the background is black.
Examples of Endospore Staining
Positive
Clostridium perfringens, C. botulinum, C. tetani, Bacillus anthracis, Bacillus cereus, Desulfotomaculum spp, Sporolactobacillus spp, Sporosarcina spp, etc.
Negative
E. coli, Salmonella spp, etc.
Quality control of Endospore Staining
Positive control: Clostridium perfringens (ATCC 13124)
Negative control: Escherichia coli (ATCC 25922)
References
- Collin County Community College District
- Portland Community College
- ASM Microbe Library: Endospore Stain Protocol
- Austin Community College
- Western Michigan University
- Sigma-Aldrich Chemie GmbH
- Bacteriological Analytical Manual 8th ed., Revision A (1998)
- H.J. Conn’s Biological Stains, 9th ed. by R.D. Lillie (1977)
- Fall 2011, Jackie Reynolds, Richland College, BIOL 2421
- Microbe Online
- Wikipedia

why is water used as decolourizer instead of alcohol in spore staining
facilitating dye penetration and prevent dehydration of cell and also water enhance hyration of spore coat
How is this staining procedure similar to spore staining technique
I am currently taking a microbiology course at the local community college. I have an unknown only known to my professor. I performed 3 staining procedures and determined that my unknown is gram-positive cocci, non-acid fast, endospore former. I thought it might be a Mycobacterium. From the information I provided, is that correct? Or is more information needed?
Hello Sagar Aryal,
I have a doubt….
Before I performe endospore stain I believe that I need that the bacteria be on endospore phase….How I do that?
I look forward yoyr answer
Monika Tanaka