- Haemophilus spp are small, pleomorphic, gram-negative bacilli or coccobacilli with random arrangements.
- A clinically important species of the genus, influenzaeis a fastidious organism which grows best at 35-37°C with ~5% CO2 and in the presence of special accessory growth factors called X and V factors.
- X factor comprises protoporphyrin IX, also called haemin or other iron-containing porphyrins. These are required for growth because X-dependent strains are unable to convert d-aminolaevulinic acid to protoporphyrin. They are heat stable.
- V factor comprises nicotinamide adenine dinucleotide (NAD) or nicotinamide adenine dinucleotide phosphate (NADP). They are heat labile.
- However, different species of Haemophilushave varying requirements for X and V growth factors.
- For example. Haemophilus parainfluenzaerequires V factor only for grow while Haemophilus ducreyi requires only X factor without need of V factor.
Objective
To differentiate among Haemophilus species based on their characteristic utilization of X and V growth factors.
Principle
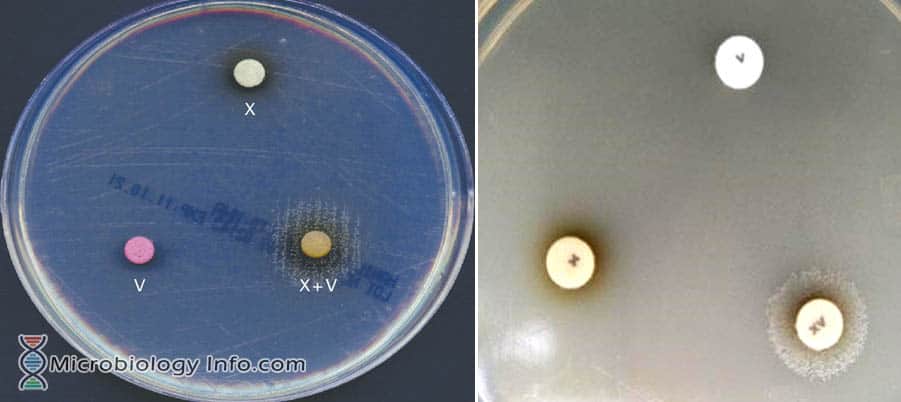
Members of the genus Haemophilus require accessory growth factors in vitro. Some Haemophilus spp. require X factor (hemin) alone, V factor (nicotinamide adenine dinucleotide [NAD]) alone, or a combination of the two. Consequently, this significant difference in growth factor requirements of Haemophilus spp. allows for their differentiation.
In the laboratory, a lawn of the test organism is streaked onto heart infusion agar, tryptic soy agar, Haemophilus agar, or nutrient agar. The impregnated disks or strips (X, V, or XV) are placed directly on the confluent inoculation, allowing diffusion of the accessory growth factor into the medium. The organisms will grow only around the disk that provides the appropriate factor for growth of the organism ie. the presence of growth around the disc but not elsewhere on the plate indicates a requirement for that particular factor. Differentiation is hence based on the presence or absence of growth around and/or between disks impregnated with factors X, V and XV.
Testing Discs
X – and V-Factor Disks are paper disks impregnated with growth factors. Each X-Factor Disk is impregnated with hemin. Each V-Factor Disk is impregnated with NAD (nicotinamide adenine dinucleotide). Each XV-Factor Disk is impregnated with a combination of hemin and NAD.
Method
- Using aseptic technique select isolated colonies of suspect colonies and prepare a suspension equivalent to a 0.5 McFarland standard.
- Dip a sterile swab into the organism suspension. Roll the swab over the entire surface of a trypticase soy agar plate. Inoculate by streaking in three different directions by rotating the plate in a 60 degree angle after each streaking. Streaking in this manner ensures confluent growth.
- Allow the surface to dry 3-5 minutes.
- Place the X, V, and XV factor disks on the agar surface. If using separate disks, place them at least 4 to 5 cm apart.
- Incubate overnight at 35°-37°C.
- Observe for growth or no growth around the disks.
Expected Results
Positive:
- Growth around the XV disk only shows a requirement for both factors.
- Growth around the V disk, no growth around the X disk, and light growth around the XV disk shows a V factor requirement.
Negative:
- Growth over the entire surface of the agar indicates no requirement for either X or V factor.
Uses
They are used for the differentiation of Haemophilus species, including Aggregatibacter aphrophilus based upon their requirements for the growth factors.
Limitations
- It is recommended that biochemical, immunological, molecular, or mass spectrometry testing be performed on colonies from pure culture for complete identification.
- Because similarities exist in growth factor requirements of Haemophilus species, it is not recommended that this procedure be the sole criterion for species identification.
- Care should be taken during inoculation of specimens onto culture media in order to prevent nutrient carryover.
References
- Tille P.M. 2014. Bailey and Scott’s diagnostic microbiology. Thirteen edition. Mosby, Inc., an affiliate of Elsevier Inc. 3251 Riverport Lane. St. Louis. Missouri 63043
- https://catalog.hardydiagnostics.com/cp_prod/content/hugo/XVFactorDisks.htm
- http://www.apsi.it/public/ufiles/smi/tp38_3_en_150108.pdf
- https://himedialabs.com/TD/DD022.pdf
Similar Posts:
- Novobiocin Susceptibility Test – Principle, Procedure, Uses and Interpretation
- List of culture media used in microbiology with their uses
- SXT Test – Principle, Procedure, Uses and Interpretation
- Haemophilus influenzae: Habitat, Cell Morphology, Cultural Characteristic, Biochemical Tests, Pathogenicity, Diagnosis and Treatment
