Novobiocin is an aminocoumarin antibiotic, produced by the actinomycete Streptomyces nivens, with antibacterial property. In 1975, Kloos and Schleifer reported a simplified scheme for differentiating coagulase-negative Staphylococcus spp. which included a novobiocin disk test.
Staphylococcus saprophyticus, a gram positive coagulase negative Staphylococci, is an uropathogenic bacterium that causes acute uncomplicated urinary tract infections, particularly in young, middle aged female patients. Unlike most other CONS, saprophyticus is rarely resistant to most antibiotics active against gram-positive organisms. S. saprophyticus is innately resistant to the antibiotic novobiocin.
Therefore, screening coagulase-negative staphylococci from urine cultures for novobiocin resistance is a reliable presumptive identification of saprophyticus.
Objective
To determine the susceptibility pattern of bacterium to antibiotic novobiocin.
Principle
Novobiocin is an antibiotic interfering with the unpackaging and repackaging of DNA during DNA replication and the bacterial cell cycle. Novobiocin binds to DNA gyrase, and blocks adenosine triphosphatase (ATPase) activity.
Susceptibility to novobiocin is determined by placing a novobiocin-impregnated paper disk on a agar plate seeded with the organism under investigation. As the organism multiplies during incubation to produce a lawn of confluent growth, cells are exposed to the antibiotic diffusing into the agar from the paper disk. If the bacteria are susceptible to novobiocin, there will be a formation of visible zone of inhibition around the disk, representing an area where the antibiotic concentration has prevented bacterial growth. No zone of inhibition around the disk represents that organism is resistant to the anitibiotic.
Hence, in the laboratory, a Mueller-Hinton agar plate is heavily seeded with the test organism to produce a confluent growth on the agar surface. After the seeding, a novobiocin antibiotic disc is applied to the agar surface. Following incubation, the sensitivity of an organism to the antibiotic is determined by the Kirby-Bauer method.
Novobiocin Disc:
Disk is prepared by impregnating 5ug of novobiocin onto high quality 6mm diameter filter paper disks (commercially available).
Method
- The test isolate should be 18-72 hours and in pure culture. Prepare a suspension of the test isolate in tryptic soy broth equal to a McFarland 0.5 standard or equivalent.
- Immerse a sterile swab into the suspension and rotate it against the side of the tube above the fluid level to remove excess inoculum.
- Using the expressed swab, inoculate a blood agar or Mueller Hinton agar plate by streaking the swab over the entire agar surface and repeat in 2 planes.
- Allow the agar surface to dry no more than 15 minutes before applying a Novobiocin Disk. With a sterile swab, prepare a lawn of growth over the entire plate by swabbing over the entire plate in 3 directions and around the edge of the plate.
- Using alcohol-dipped and flamed forceps, aseptically apply a novobiocin antibiotic disc to the surface of each inoculated plate. Gently press the discs down with sterile forceps to ensure that they adhere to the agar surface.
- Incubate plate aerobically for 18 to 24 hours at 35 to 37°C.
- Measure the diameter of the zone of inhibition using sliding calipers or a metric ruler.
Expected Results
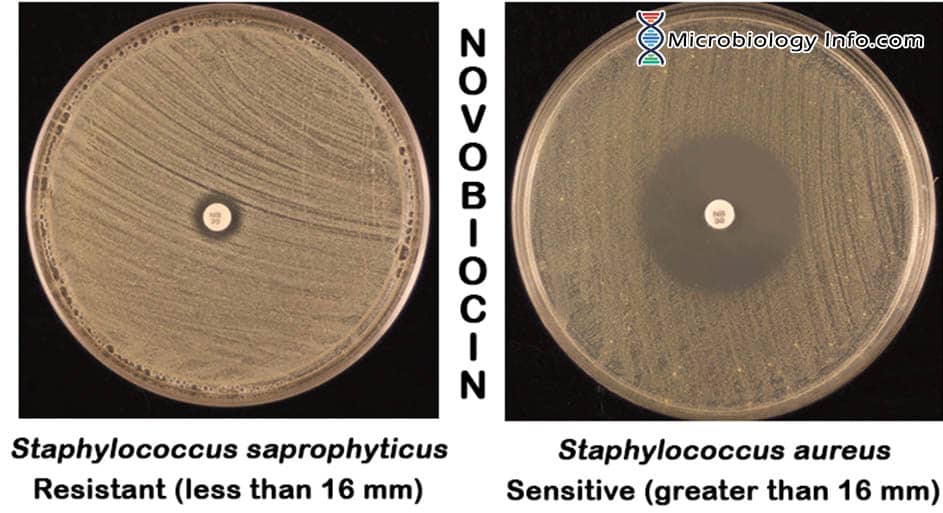
- Positive – A zone of inhibition greater than 16mm indicates that the organism is sensitive to the antibiotic.
- Negative – A zone of inhibition less than or equal to 16mm is indicative of novobiocin resistance.
Uses
- The CoNS have been subdivided into two groups based on their novobiocin susceptibility pattern. The CoNS group that demonstrates novobiocin susceptibility includes epidermidis, S. capitis, S. haemolyticus, S. hominis subsp. hominis, S. lugdunensis, S. saccharolyticus, S. warneri, and other species.
- The novobiocin resistant group consists of such species as cohnii, S. kloosii, S. saprophyticus, and S. xylosus.
- It is useful for presumptively distinguishing Staphylococcus saprophyticusfrom other coagulase-negative staphylococci in clinical specimens.
Limitations
- Testing should be performed on isolated colonies of aerobic, catalase-positive, coagulase-negative gram positive cocci.
- It is recommended that biochemical, immunological, molecular, or mass spectrometry testing be performed on colonies from pure culture for complete identification.
- The novobiocin disk is not helpful and can give misleading results if it is performed on isolates other that those from urinary specimens.
- Occasional human isolates that are not S. saprophyticus, S. cohnii subsp., or S. xylosis may also be resistant to novobiocin
References
- Murray, P.R., E.J. Baron, J.H. Jorgensen, M.L. Landry, and M.A. Pfaller. 2007. Manual of Clinical Microbiology. 9th ed. ASM Press, Washington, D.C.
- https://catalog.hardydiagnostics.com/cp_prod/content/hugo/NovobiocinDiffDisks.htm
- https://watermark.silverchair.com/labmed160422.pdf
- www.vumicro.com/vumie/help/VUMICRO/Novobiocin_Susceptibility.htm
- https://pubchem.ncbi.nlm.nih.gov/compound/novobiocin
