Gelatin is a protein derived from the connective tissues of vertebrates, that is, collagen. It is produced when collagen is boiled in water. Gelatin hydrolysis detects the presence of gelatinases. Gelatinases are proteases secreted extracellularly by some bacteria which hydrolyze or digest gelatin. The production of gelatinases is used as a presumptive test for the identification of various organisms, including Staphylococcus sp., Enterobacteriaceae, and some gram-positive bacilli.
Principle
This test is used to determine the ability of an organism to produce extracellular proteolytic enzymes (gelatinases) that liquefy gelatin, a component of vertebrate connective tissue.
The reaction occurs in two sequential steps: in first reaction gelatinases hydrolyze gelatin into polypeptides and then polypeptides are further converted into amino acids. The amino acid is taken up by the cell and used for metabolic purposes.
The presence of gelatinases is detected using a nutrient gelatin medium. When an organism produces gelatinase, the enzyme liquefies the growth medium by hydrolyzing gelatin present in the medium.
Media:
Nutrient gelatin medium
Enzymatic digest of gelatin (5 g), beef extract (3 g), gelatin (120 g), per 1000 mL, pH 6.8.
Method
There are several methods for determining gelatinase production, all of which make use of gelatin as the substrate. The standard and most commonly employed method is the nutrient gelatin stab method.
- Inoculate the gelatin deep with 4 to 5 drops of a 24-hour broth culture.
- Incubate at 35°-37°C in ambient air for up to 14 days.
Note: Incubate the medium at 25°C if the organism grows better at 25°C than at 35°C.
- Alternatively, inoculate the gelatin deep from a 24-hour-old colony by stabbing four or five times, 0.5 inch into the medium.
- Remove the gelatin tube daily from the incubator and place at 4°C to check for liquefaction.
Note: Do not invert or tip the tube, because sometimes the only discernible liquefaction occurs at the top of the deep where inoculation occurred.
- Refrigerate an un-inoculated control along with the inoculated tube. Liquefaction is determined only after the control has hardened (gelled).
Nutrient gelatin plate method
- Stab-inoculate a heavy inoculum of an 18- to 24-hour-old test bacteria onto culture plates prefilled with nutrient gelatin (23 g/liter nutrient agar, 8 g/liter gelatin).
- Incubate inoculated nutrient gelatin plates at 35oC for 24 hours.
Note:
In some cases, plates are flooded with saturated ammonium sulfate to precipitate unhydrolyzed gelatin, making the clear zones easier to see. Results are often observed within 5 to 10 minutes after flooding with saturated ammonium sulfate.
Expected Results
- Positive: Partial or total liquefaction of the inoculated tube (the control tube must be completely solidified) at 4°C within 14 days. On plates, gelatin hydrolysis is indicated by clear zones around gelatinase-positive colonies.
- Negative: Complete solidification of the tube at 4°C. On plates, no clear zones around colonies are observed.
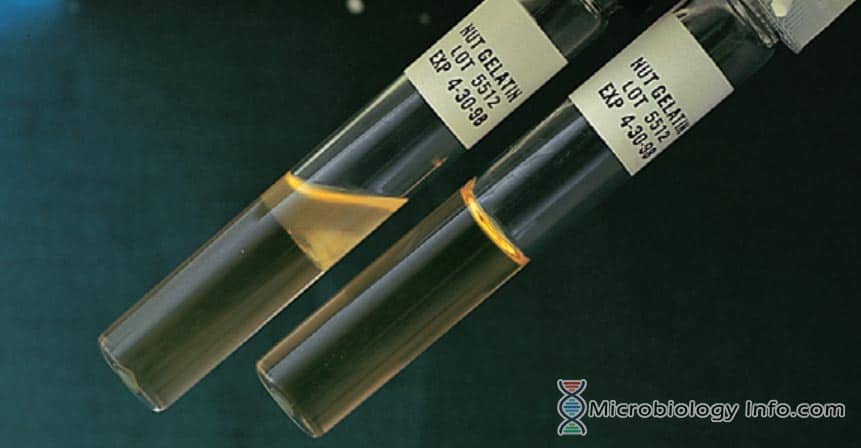
Gelatin hydrolysis. A, Positive; note liquefaction at top of tube. B, Uninoculated tube.
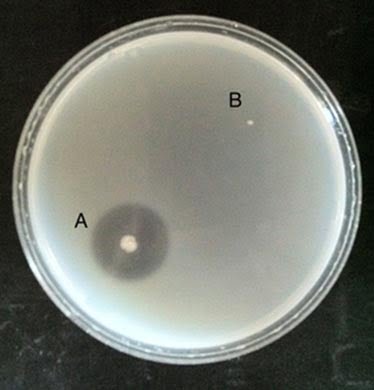
Gelatin hydrolysis. A, Positive B, Negative
Uses
- This test is used to determine the ability of an organism that produce gelatinases.
- This test is helpful in identifying and differentiating species of Serratia, Proteus, Bacillus, Clostridium, Pseudomonas and Flavobacterium.
- This test differentiates pathogenic Staphylococcus aureus which is gelatinase-positive from non-pathogenic epidermidis which is gelatinase negative.
- This test can be used to differentiate Serratia and Proteus species which are gelatin positive from other members of Enterobacteriaceae family.
- Bacillus anthracis, B. cereus and several other members of the genus are gelatinase-positive, as are Clostridium tetani and perfringens.
Limitations
- Some organisms may grow poorly or not at all in this medium.
- Gelatin is liquid above 20°C; therefore determination of results must be completed following refrigeration.
- Gelatinase usually acts at the surface of the medium. Shaking the tube while it is warm may result in false-negative interpretation.
References
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- www.asmscience.org/content/education/protocol/protocol.3776
- www.austincc.edu/microbugz/gelatinase_test.php
- www.biologypractical.com/gelatin-hydrolysis-test-principle-procedure/
Similar Posts:
- Widal Test- Introduction, Principle, Procedure, Interpretation and Limitation
- Bile Solubility Test- Principle, Reagents, Procedure and Result Interpretation
- Oxidase Test- Principle, Uses, Procedure, Types, Result Interpretation, Examples and Limitations
- Urease Test- Principle, Media, Procedure and Result
