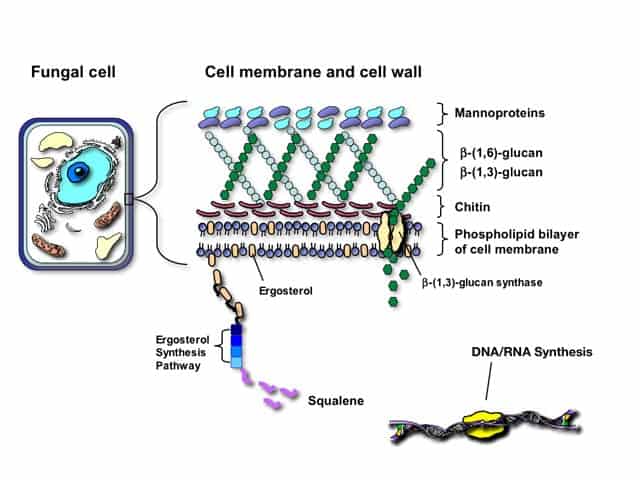
Fungal cell membranes have a unique sterol, ergosterol, which replaces cholesterol found in mammalian cell membranes. An antifungal agent is a drug that selectively eliminates fungal pathogens from a host with minimal toxicity to the host.
1. Polyene antibiotics
The poleyenes possess a macrocyclic ring, one side of which has a several conjugated double bonds and is highly lipophilic while the other side is hydrophilic with many OH groups. A polar aminosugar and a carboxylic acid group are present at one end in some. They are all soluble in water and unstable in aqueous medium.
A. Amphotericin B
- Amphotericin B is a naturally occurring polyene macrolide antibiotic produced by Streptomyces nodosus.
- Amphotericin B is prototype of polyene antibiotics and have high affinity for ergosterol present in fungal cell membrane.
- It then gets combined with the membrane and get inserted into the membrane and several molecules together orient themselves in such a way as to form the micropore.
- The hydrophilic side forms the interior of the pore through which ions, aminoacids and other water soluble substances move out.
- The cell permeability is markedly increased and due to this the cell death occurs.
B. Nystatin
- Nystatin is obtained from noursei and is similar to Amphotericin B in antifungal action and other properties.
- It binds to fungal cell membrane (Ergosterol) and forms pores.
- This alters permeability & transport and as a result, cell death occurs.
2. Heterocyclic benzofuran
Griseofulvin
- It was one of the early antibiotics extracted from Penicillium griseofulvum.
- It is active against most dermatophytes including epidermophyton, trichophyton , microsporum but not against Candida and other fungi causing mycosis.
- It is fungistatic in nature.
- It interferes with mitosis-multinucleated and stunned fungal hyphae result from its action.
- It causes abnormal metaphase configuration, however doesn’t cause metaphase arrest, rather the daughter nuclei fail to move apart or move only a short distance.
- It does not inhibit polymerization of tubulin but somehow disorients the microtubules.
3. Antimetabolite
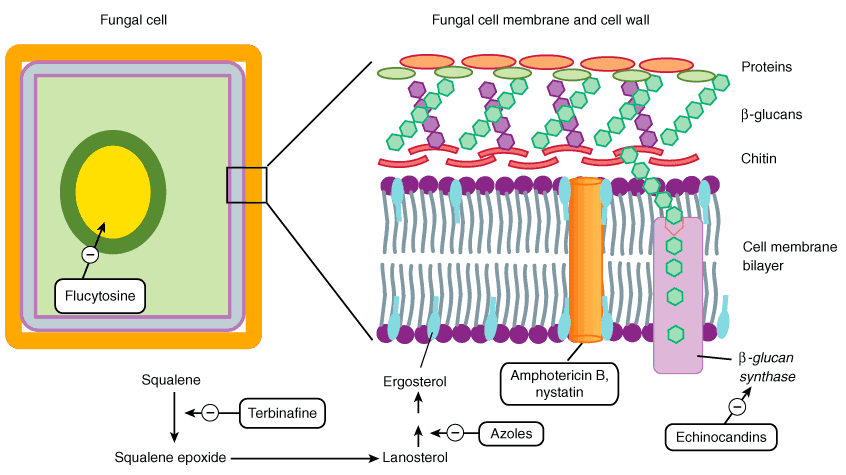
Flucytosine
- It is a pyrimidine antimetabolite and inactive as such.
- Flucytosine is taken up by fungal cells via the enzyme cytosine permease.
- 5-FC is then converted by a series of steps to 5-fluorodeoxyuridine 5’-monophosphate.
- This false nucleotide inhibits thymidylate synthase, thereby depriving the organism of thymidylic acid, an essential DNA component.
- The unnatural mononucleotide is further metabolized to a trinucleotide (5-fluorodeoxyuridine 5’-triphosphate) and is incorporated into fungal RNA, where it disrupts nucleic acid and protein synthesis.
4. Echinocandins
- Echinocandins interfere with the synthesis of the fungal cell wall.
- It is Glucan synthesis inhibitor.
- It nhibits 1,3- beta glucan synthase, an enzyme important in fungal cell wall synthesis and subsequently inhibit the synthesis of beta glucan in the fungal cell wall.
- Disruption of the fungal cell wall leads to cellular osmotic instability and cell death.
5. Terbinafine
- It is synthetic allylamine and orally active.
- It is used for treating dermatophytoses, especially onychomycosis.
- It interferes with ergosterol biosynthesis by inhibiting the fungal enzyme squalene epoxidase rather than interacting with the P450 system.
- Acting as a structural analogue of squalene, terbinafine causes the accumulation of this unsaturated hydrocarbon, and a decrease in ergosterol in the fungal cell membrane.
- The accumulation of toxic amounts of squalene result in the death of the fungal cell.
6. Azoles
- The azole antifungal drugs act by inhibiting the synthesis of the sterol components of the fungal membrane.
- Azoles are predominantly fungistatic.
- They inhibit C-14 α-demethylase (a cytochrome P450 [CYP450] enzyme), thereby blocking the demethylation of lanosterol to ergosterol, the principal sterol of fungal membranes.
- This inhibition disrupts membrane structure and function, which then inhibits fungal cell growth.
- Azoles are primarily divided into two groups: imidazoles and triazoles.
- Clotrimazole, Econazole, Miconazole, Ketoconazole fall under imidazoles group and Fluconazole, Itraconazole, Posaconazole and Voriconazole fall under triazoles group
- The imidazoles and triazoles cause rapid defects in fungal membrane integrity due to reduced levels of ergosterol, with loss of cytoplasmic constituents leading to similar effects to the polyenes.
- They impair ergosterol synthesis leading to cascade of membrane abnormalities in the fungus.
7. Topical antifungals
A. Ciclopirox
- Ciclopirox inhibits the transport of essential elements in the fungal cell, disrupting the synthesis of DNA, RNA, and protein.
- Ciclopirox is active against Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, Microsporum canis, Candida albicans, and Malassezia furfur.
B. Tolnaftate
- Tolnaftate distorts the hyphae and stunts mycelia growth in susceptible fungi.
- Tolnaftate is active against Epidermophyton, Microsporum, and Malassezia furfur.
References
- Denyer S.P, Hodges N, Gorman S.P and Gilmore B.F. 2011. Hugo and Russell’s Pharmaceutical Microbiology. Eighth edition. A John Wiley & Sons, Ltd. Publication. Page no.191-193.
- Clark M.A, Finkel R, Rey J.A, and Whalen K. 2012. Lippincott’s Illustrated Reviews: Pharmacology. Fifth edition. Lippincott Williams & Wilkins, a Wolters Kluwer business. 351 West Camden Street Two Commerce Square Baltimore, MD 21201. Page no.429-440.
- Tripathi K.D. 1994. Essentials of Medical Pharmacology. Third edition. Jaypee Brothers Medical Publishers (P) Ltd. B-3 EMCA House 23/23B, Ansari Road, Daryaganj, New Delhi 110 002, India. Page no.707-716.