Habitat
- Histoplasma capsulatum is a dimorphic ascomycete that grows in its hyphal form in soil and bird and bat guano.
- The natural habitat of capsulatum is the soil and has also been recovered most frequently from soil material contaminated with bird or bat droppings.
- Unlike bats, birds do not become infected with capsulatum and their droppings serve as a nutrient source for the fungus which already present in the soil.
- Bats are capable of depositing capsulatum with their droppings and can distribute the fungus to distant from an existing natural source.
- The fungus has been found in poultry house litter, caves, areas harboring bats, and in bird roosts.
- Soil samples from sites where birds have roosted have been found to remain contaminated for at least 10 years even after the roost has been cleared.
Morphology
- capsulatum is a thermally dimorphic fungus which exists as a mycelial form at ambient temperatures and grows as small round budding yeast cells at body temperature in mammals.
- There are two varieties of capsulatum recognized: var. capsulatum and var. duboisii where H. capsulatum var duboisii is the cause of African histoplasmosis.
- The two varieties are indistinguishable in their mould forms, but there is a difference in their parasitic forms.
- The cells of the tissue form of duboisii are much larger and comprised of thicker walls than those of var. capsulatum.
- Growth on Sabouraud dextrose agar at 25ºC shows a white to brown colony, cottony mycelium after 2 to 3 weeks.
- The mold phase is characterized by thin, branching, septate hyphae that produce microconidia and a very distinct spore called a tuberculate macroconidium.
- Grown at 37ºC shows the budding yeast form.
- The yeast cell is 2-4 µm in diameter and slightly oval in shape and found exclusively within macrophages.
Epidemiology
- Histoplasma capsulatum is a dimorphic fungal pathogen of worldwide importance that causes a broad spectrum of disease activity.
- Histoplasmosis is the most prevalent mycosis in North America and more often reported in the areas surrounding the Mississippi and Ohio rivers.
- Infection occurs via exposure to dust or soil for prolonged periods of time including activities which disturb bird and bat guano which increase the risk of infection.
- In Ohio and Mississippi River valleys, capsulatum is endemic and approximately 80 % of the general population tested showed hypersensitivity to Histoplasma capsulatum with young adults being the largest group to be affected.
- Histoplasmosis is also found in Central and South America, Australia, eastern Asia, and the tropical areas of Africa.
- Although the course of infection is mild in immunocompetent individuals, the individuals with immunocompromised status are at an increased risk of infection, and hold an increased severity of infection with a higher mortality rate.
Virulence factors
1. Cell wall a-1,3 glucan
- a-(1,3)-glucan is a polysaacharide layer found on the yeast cell surface that conceals cell surface ß glucans, which have antigenic properties.
2. Heat shock proteins
- Two types of heat shock proteins are present and play a significant role in the pathogenesis.
- Heat shock protein 60 (HSP60) that plays important roles in chaperoning intracellular proteins and supervising adequate protein folding and also considered as an essential surface molecule, mediating the recognition and phagocytosis of the yeast by macrophages.
- HSP60 acts as a ligand for CD11/CD18 macrophage receptor and couples with the CR3 receptor which is followed by rapid ingestion of the yeast.
- The interaction between Histoplasma and macrophage through HSP60 binding to CR3 results only in a mild host immune reaction and also allows the yeast to survive and replicate inside the host cells.
- Additionally, Heat shock protein 82 (HSP82) is another important molecule that helps in normal development of capsulatum and also participates in the response to cellular stresses.
- It binds to a variety of cellular proteins, keeping them inactive until they have reached their proper intracellular location or have received the proper activation signal.
3. Yeast Phase Specific (YPS3) gene
- YPS3 is the encoded protein found both as a fungal cell wall constituent and as a secreted molecule whose exact function is still unknown but it is found as a virulence factor contributing in the yeast pathogenesis.
4. Cell wall melanin
- The production of melanin and melanin like pigments by capsulatum conidia and yeast decreases the susceptibility of the fungus to antifungal agents such as amphotericin B and caspofungin.
- In addition, melanin can abrogate the potency of certain host defense mechanisms, such as free radicals and microbicidal peptides.
5. Calcium-binding protein (CBP)
- Calcium-binding proteins are secreted by the fungal cells during the yeast-phase of intracellular growth within the macrophage.
- It facilitates optimal phagolysosomal conditions for yeast growth.
6. Siderophores
- Sidephores support the intracellular growth of the yeast.
7. Histone 2B (H2B)
- Histones are mainly intracellular components which plays role in the pathogenesis of the capsulatum.
Pathogenesis
- Histoplasma capsulatum exists in the saprophytic stage as a mycelial form consisting of hyphae which bear both macroconidia and microconidia.
- The portal of entry of capsulatum is through inhalation of aerosolized of 2–6?µm diameter microconidia which initially enters into the terminal bronchioles and then into alveoli of the lung.
- The incubation period is 1-3 weeks after initial infection and upon re- infection, symptoms present in a shorter period of time 4-7 days.
- The inhaled microconidia undergo morphogenesis which is initiated after infection and microconidia develop into a 2–4?µm oval yeast form which is responsible for the pathogenesis of histoplasmosis.
- Alveolar macrophages then phagocytosed the yeast form and they multiply within the macrophages.
- Macrophages have a central role in the interaction between the yeast and the host, although possess a dual nature.
- Macrophages firstly provide an environment for fungal replication and dissemination, and then subsequently act as the final effector cells to remove the organism from the host.
- Binding of capsulatum yeasts to human monocyte-derived macrophages is temperature-dependent being optimum at 37ºC, and requires the presence of the divalent cations Ca and Mg.
- Macrophage renders the first line of defense during infection, as they rapidly phagocytose the inhaled conidia and transforming yeast cells, and the infected macrophage subsequently activate effector T cells and enhance the release of Th1-associated proinflammatory cytokines such as IL-12, IFN-?, and TNF-a.
- The commonest action shown by macrophages to neutralize pathogen include deprivation of zinc and iron, followed by production of superoxide, nitric oxide, lysosomal hydrolysases and cationic peptides which destroy the organism.
- The yeast form however find alternative for survival.
- capsulatum yeast cells are able to regulate the pH of the phagolysosomes at a neutral pH (pH 6.5), where lysosomal hydrolases show decreased activity.
- Hence, the capsulatum yeast cells manage to survive within the macrophages and even replicate inside it.
- The dividing yeasts tend to destroy the alveolar macrophages and subsequently are ingested by other resident alveolar marophages and by inflammatory phagocytes recruited to the loci of infection.
- The repetition of this cycle results in spread of infection to hilar lymph nodes where they gain access to the blood circulation for dissemination to various organs.
- Macrophages assist in spreading the organism via lymphatics and the blood to the adjacent lymph nodes and throughout the reticuloendothelial system (liver, spleen, lymph nodes, and bone marrow).
Clinical manifestations
1. Acute Pulmonary Histoplasmosis
- The infection is asymptomatic in at least 90% of cases when there is low-inoculum exposure.
- When symptoms are present, it is generally mild and often characterized by fever, cough, and chest pain.
- Most patients recover in a few weeks, but some individual experience prolonged fatigue.
- Heavy exposure, on the other hand, causes diffuse pulmonary involvement, which is often accompanied by respiratory failure.
- In some cases, patients with acute histoplasmosis may experience rheumatological syndromes characterized by arthritis or arthralgia (joint pain), and erythema nodosum.
- The other inflammatory complication found is pericarditis.
- Both pericarditis and rheumatological syndromes occur in less than 10% of cases.
- On chest radiograph, it shows small scatter, nodular infiltrates and lymphnode enlargement.
- In case if reinfection, the incubation time is shorter (< 1 week).
2. Chronic Pulmonary Histoplasmosis
- Chronic pulmonary histoplasmosis occurs in individuals with underlying bullous lung disease and symptoms last for more than 6 weeks.
- It is characterized by recurrent pulmonary illnesses accompanied by complications like progressive lung infiltrate, fibrosis, cavitation and respiratory deterioration.
- Productive cough, fever, chest pain, fatigue, weight loss and haemoptysis are common symptoms.
- Initially it manifests itself as a transient, segmental pneumonia that recover after sometime without treatment, but often progresses to fibrosis and cavitation with destruction of significant amounts of lung tissue.
- If the infection is left unresolved, it causes destruction of significant amount of lung tissue and death may occur from progressive lung failure.
3. Disseminated Histoplasmosis
- Hematogenous dissemination from the lungs to other tissues occurs in some cases during the acute infection but is rarely recognized clinically.
- Progressive disseminated disease occurs in about 1 in 2000 acute infections, mostly in those patients with immunosuppressed status or those at the extremes of age.
- Common clinical manifestations include fever, weight loss, and respiratory symptoms.
- Laboratory tests usually show anemia, leukopenia, thrombocytopenia, and elevated hepatic enzymes and bilirubin.
- Shock with hepatic, renal, and respiratory failure may complicate severe cases.
- CNS diseases may occur as manifestations of widely disseminated infection in about 10–20% of patients presenting meningitis and focal brain or spinal cord lesions.
- Other frequent sites of dissemination include the oral mucosa, gastrointestinal tract, skin, kidneys, and adrenal glands.
4. Histoplasmoma
- In very rare cases, patients may develop a slowly enlarging pulmonary nodule, called as an ‘enlarging histoplasmoma’ causing concern about neoplasia.
- Lesions range from 8 to 35 mm in diameter and enlarge slowly over a period of time.
- Histologically, they are characterized by a necrotic center surrounded by a fibrous-like capsule and organism may be present in the center.
5. African histoplasmosis
- African histoplasmosis is caused by Histoplasma capsulatum var. duboisii and is characterized by presence of granulomatous lesions in the skin, subcutaneous tissues, lymph nodes and bones.
- The lungs and other internal organs are rarely involved.
- Cutaneous lesions are common and multiple papular lesions often develop on the face and trunk while nodular lesions are less common.
- The cutaneous lesions are polymorphic and presented as papules, ulcers, nodules, abscesses, fistulas or pigmentary changes.
- The papules and nodules present have a characteristic hyperpigmented halo around them and both nodules and papules often enlarge and ulcerate.
- In case of subcutaneous lesions, abscesses are frequently encountered which are filled with thick, yellowish pus that is always rich in large yeast forms, characteristic of capsulatum var. duboisii.
- Abscesses may discharge through fistulas in the skin and fistulas may also result from the inflamed lymph nodes.
- Osteomyelitis occurs in about 30% of patients with African histoplasmosis and the bones involved are mainly the spines, ribs, cranial bones, sternum, the long bones of arms and legs.
- Multiple lesions are often found and the lesions are often painless.
- The infection may spread into contiguous joints causing arthritis, or into adjacent soft tissue, causing a purulent subcutaneous abscess.
Laboratory diagnosis
Specimens: Sputum, Blood, Bone marrow, Urine, Oral lesion scraping, Lymph node biopsy, Broncho Alveolar Lavage (BAL), pus.
1. Direct microscopic examination
- Smears of sputum or pus are stained by Giemsa or Wright’s stains and examined microscopically.
- Skin scrapings, exudates and body fluids should be examined using 10% KOH and Parker ink or calcofluor white mounts.
- Tissue sections should be stained using PAS (Periodic Acid Schiff) digest, Grocott’s methenamine silver (GMS) or Gram stain.
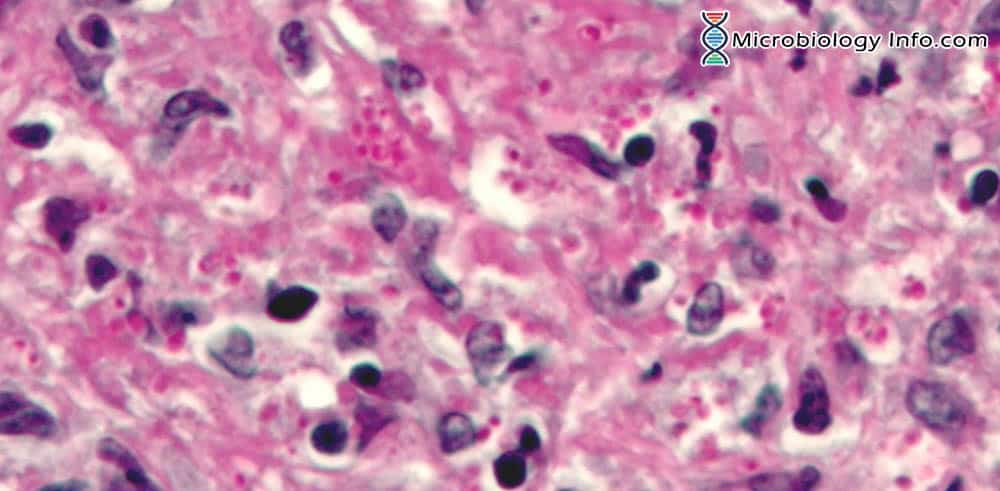
- Specimens can be evaluated directly for the yeast form of Histoplasma capsulatum and appears as small oval intracellular yeast cells packed within macrophages or monocytes.
- Blood and bone marrow smears may also be positive particularly in patients suffering from AIDS. So stains that can be used for blood and bone marrow include the Giemsa and Wright’s stains.
2. Culture
- It is most useful for specimens from patients with disseminated or chronic pulmonary histoplasmosis.
- Specimens are inoculated on SDA media and incubated at 25ºC for 6-12 weeks.
- The colonies are initially smooth, but become filamentous, cottony, and brownish on the surface and yellowish on reverse with the age.
- capsulatum is mycelial at 25°C where it grows with hyaline hyphae that have tuberculated macroconidia and smooth-walled spherical, pyriform or cigar shaped microconidia ranging in size from 2µm to 6µm in diameter.
- At 37°C, yeast forms predominate that are generally ovoid thick-walled cells.
- The hyphae are hyaline and septate.
- Its macroconidia are unicellular, hyaline, thick-walled, and tuberculate (meaning bumpy or knobby).
- Microconidia are unicellular, and hyaline, with a smooth or rough wall.
Culture of yeast form
- The conversion from the mold phase to the yeast phase is necessary for accurate diagnosis of capsulatum which is achieved by subculturing in a enriched media such as blood agar or Brain-heart infusion agar (BHI) with cystein and incubated at 35ºC in ambient air for 2-4 weeks.
- Colony has a creamy texture with cream color on its surface and reverse.
- Microscopically the yeast morphology has small budding yeast that measure 2-4 um in length.
- Another clue is to correlate findings with the pathological reports of tissue biopsies that would have the intracellular yeast cells.
3. Histoplasmin skin test
- Histoplasmin skin test is similar to tuberculin test.
- Skin test becomes positive 2-4 weeks after a person is injected.
- This method evaluates the reactivity of the patients with histoplasmosis when challenged intradermally with fungal proteins.
- It is not usually recommended for diagnosis because it doesn’t help to differentiate past and present infection.
4. Serology
a) Antibody detection
- The two routine methodologies used for the detection of antibody include complement fixation and immunodiffusion.
- However this method should not be used in patients with the disseminated form of histoplasmosis with respect to the fact that there is an increase in false-negative results.
- Serologic diagnosis is mainly focused on the identification of anti-H and anti-M antibodies which can be detected using histoplasmin.
- Histoplasmin is the antigenic extract obtained from mycelial culture of capsulatum.
- Beside immunodiffusion and complement fixation test, antibodies to capsulatum can also be detected by immunoenzimatic assays such as Western blot and ELISA.
b) Antigen detection
- In the diagnosis of histoplasmosis, antigen detection is more effective than antibody detection method.
- During infection with capsulatum, antigen can be released by the fungal cells and detected in body fluids such as serum, pleural fluid, bronchoalveolar lavage fluid, cerebrospinal fluid and urine which can be detected by radioimmunoassay (RIA).
- In addition to RIA, enzyme-linked immunosorbent assays (ELISA) can also be used for the detection of a polysaccharide antigen from capsulatum (HPA).
5. Molecular diagnosis
- Molecular methods used for the identification of fungal isolates include species-specifics DNA.
- The use of chemiluminescence-labeled DNA probes for the detection of specific sequences of rRNA could detect capsulatum directly on excised tissues, such as excised heart valves from patients with Histoplasma endocarditis.
- Polymerase Chain Reaction (PCR) diagnosis based on the amplification of fungal gene sequences is a powerful tool for identifying fungal isolates in invasive mycoses.
Treatment
- Itraconazole is commonly used antifungal medication to treat mild to moderate cases of
- In case of severe infection, patient should be treated with amphotericin B.
- Since the drug can be very toxic to the kidneys and liver, patients should be switched to a less toxic oral drug, such as itraconazole when the condtion is stable.
Prevention
- Avoid exposure to areas like poultry houses, caves, other areas harboring bats, and near bird roosts.
- Spraying contaminated surfaces thoroughly with water before digging soil or working in an area that could harbor the fungus that causes histoplasmosis. Spraying water helps to prevent spores from being released into the air.
- Wearing an effective face mask or respiratory mask is recommended which provide protection against exposure.
References
- Richardson M.D and Warnock D.W. (2003). Fungal Infection- Diagnosis and Management. Third edition. Blackwell Publishing Ltd. Page 264-278.
- Center for Disease Control and Prevention (CDC)
- Horwath, M. C., Fecher, R. A., & Deepe, G. S. (2015). Histoplasma capsulatum, lung infection and immunity. Future microbiology, 10(6), 967-75.
- Mihu M.R and Nosanchuk J.D. (2012). Histoplasma Virulence and Host Responses. International Journal of Microbiology, vol. 2012, Article ID 268123, 5 pages https://doi.org/10.1155/2012/268123.
- Gugnani H. C. (2000). Histoplasmosis in Africa: A Review. Indian J Chest Dis Allied Sci; 42 : 271-277.
- Bracca, A., Tosello, M. E., Girardini, J. E., Amigot, S. L., Gomez, C., & Serra, E. (2003). Molecular detection of Histoplasma capsulatum var. capsulatum in human clinical samples. Journal of clinical microbiology, 41(4), 1753-5.
- Guimarães, A. J., Nosanchuk, J. D., & Zancopé-Oliveira, R. M. (2006). Diagnosis of Histoplasmosis. Brazilian journal of microbiology : [publication of the Brazilian Society for Microbiology], 37(1), 1-13.
Similar Posts:
- Cryptococcus Neoformans – Habitat, Morphology, Epidemiology, Virulence Factors, Treatment + More
- Alternaria Alternata – Habitat, Morphology, Epidemiology, Pathogenesis, Laboratory Diagnosis + more
- Mode of Action of Antifungal Drugs
- Scrub Typhus- Etiology, Epidemiology, Symptoms, Pathogenesis, Diagnosis and Treatment