The MRS formulation was developed by de Man, Rogosa and Sharpe to replace a variable product (tomato juice) and, at the same time, provide a medium which would support good growth of lactobacilli in general. Hence, MRS agar and broth given to their superiority are commonly employed for culturing and identifying of Lactic acid bacteria especially the Lactobacillus spp.
“Lactic acid bacteria” includes species of the following genera: Lactobacillus, Streptococcus, Pediococcus and Leuconostoc. All these species can produce lactic acid in considerable amounts.
They are Gram-positive, catalase and oxidase negative and are fastidious in their nutritional requirements. Growth is enhanced considerably by microaerobic conditions.
MRS medium is selective for lactobacilli but some growth of leuconostocs and pediococci may occur. Selectivity can be altered by pH adjustment. Lactobacilli will tolerate lower pH levels than streptococci (pH 5.0-6.5) with pediococci and leuconostocs growing best within this range.
Objective
To determine whether an organism forms gas during glucose fermentation.
Principle
The MRS broth contains sources of carbon, nitrogen, and vitamins to support the growth of lactobacilli and other organisms. Enzymatic Digest of Animal Tissue, Beef Extract, and Yeast Extract are the carbon, nitrogen, and vitamin sources used to satisfy general growth requirements in Lactobacilli MRS Broth. Dextrose is the fermentable carbohydrate. Sodium Acetate is an inhibitory agent. Sodium Acetate and Ammonium Citrate act as selective agents as well as energy sources. Potassium Phosphate is the buffering agent. Magnesium Sulfate and Manganese Sulfate provide cations used in metabolism. Polysorbate 80 is a surfactant, facilitating uptake of nutrients by lactobacilli.
When an organism is inoculated into the broth medium, those that are able to ferment the dextrose sugar, overcome the selective agents and utilize other nutrients provided show luxuriant growth which is considered a positive result. Some organism may also be able to produce gas for which a Durham tube may be placed to differentiate such gas-producers from the rest of the orgnaisms (eg. Leuconostoc sp. from Lactobacillus spp.).
Media:
Enzymatic digest of animal tissue (10 g), beef extract (10 g), yeast extract (5 g), dextrose (20 g), NaC2H3O2 (5 g), polysorbate 80 (1 g), KH2PO4 (2 g), ammonium citrate (2 g), MgSO4 (0.1 g), MnSO4 (0.05 g), per 1000 mL, pH 6.5.
Method
- Inoculate MRS broth with an 18- to 24-hour culture from agar or broth.
- Incubate 24 to 48 hours at 35°-37°C in ambient air.
- Examine the test tube for turbidity and gas production.
Expected Results
- Positive: Growth with marked turbidity of the broth, gas production indicated by a bubble in the Durham tube (Leuconostoc spp.) Growth resulting turbidity, no gas production (Lactobacillus spp)
- Negative: No growth (no turbidity) or gas production
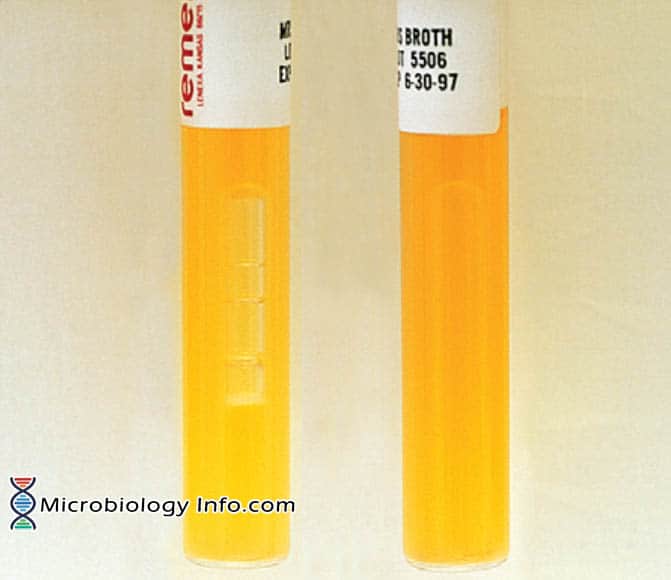
MRS broth. A, Positive; gas production by Leuconostoc sp.(Left).
B, Positive: growth, no gas production by Lactobacillus sp.(Right)
Uses
- It is used for the identification of some Lactobacillus spp. and Leuconostoc sp. which produce gas.
- The test is a part of the confirmatory tests performed on organisms isolated on MRS Agar.
- Commonly performed to identify Lactobacilli from oral cavity, dairy products, foods, faeces and other sources.
Limitations
- It is recommended that biochemical, immunological, molecular, or mass spectrometry testing be performed on colonies from pure culture for complete identification.
- Due to varying nutritional requirements, some strains may be encountered that grow poorly or fail to grow on this medium.
- Organisms other than lactobacilli may grow in this medium. Isolates must be confirmed as lactobacilli by appropriate biochemical testing.
References
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/LactobacilliMRSBroth.html
- www.labm.com/products/m-r-s-broth.asp
- https://foodsafety.neogen.com/pdf/acumedia_pi/7406_pi.pdf
- www.scharlabmagyarorszag.hu/katalogus/02-135_TDS_EN.pdf
- https://www.bd.com/europe/regulatory/Assets/IFU/Difco_BBL/288110.pdf

I crave tomato juice when I’m on a flare up
And running low ! This actually makes sense now