The Kligler’s Iron Agar test employs a medium for the identification of Enterobacteriaceae, based on double sugar fermentation and hydrogen sulphide production. In 1918, Kligler described a medium for detection of H2S and differentiation of Salmonella spp. Bailey and Lacey further modified the medium by substituting phenol red indicator for Andrade indicator. This medium became known as KIA. It is recommended for determination of H2S production by enteric gram-negative bacilli and for detection of H2S produced by some strains of Pseudomonas.
Objective
To differentiate organisms by demonstrating hydrogen sulfide production and the fermentation of dextrose and lactose.
Principle
- Kligler Iron Agar, in addition to peptone, HM peptone B and yeast extract, contains lactose and glucose (dextrose), which enables the differentiation of species of enteric bacilli. Phenol red is the pH indicator, which exhibits a color change in response to acid produced during the fermentation of sugars.
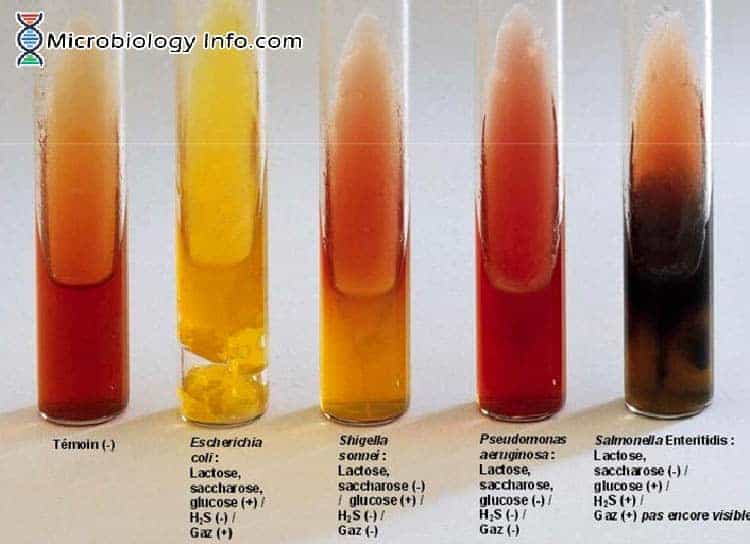
- Fermentation of dextrose results in production of acid, which turns the indicator from red to yellow. Since there is little sugar i.e. dextrose, acid production is very limited and therefore a reoxidation of the indicator is produced on the surface of the medium, and the indicator remains red. However, when lactose is fermented, the large amount of acid produced, avoids reoxidation and therefore the entire medium turns yellow.
- The combination of ferrous sulphate and sodium thiosulphate enables the detection of hydrogen sulfide production, which is evidenced by a black color either throughout the butt, or in a ring formation near the top of the butt.
- Lactose non-fermenters (e.g., Salmonella and Shigella ) initially produce a yellow slant due to acid produced by the fermentation of the small amount of glucose (dextrose). When glucose (dextrose) supply is exhausted in the aerobic environment of the slant, the reaction reverts to alkaline (red slant) due to oxidation of the acids produced. The reversion does not occur in the anaerobic environment of the butt, which therefore remains acidic (yellow butt).
- Lactose fermenters produce yellow slants and butts because of lactose fermentation. The high amount of acids thus produced helps to maintain an acidic pH under aerobic conditions. Tubes showing original color of the medium indicates the fermentation of neither glucose (dextrose) nor lactose.
- Gas production (aerogenic reaction) is detected as individual bubbles or by splitting or displacement of the agar by the formation of cracks in the butt of the medium.
Media:
In Gms / Litre : Peptone 15.000gm, HM Peptone B # 3.000 gm, Yeast extract 3.000 gm, Proteose peptone 5.000 gm, Lactose 10.000gm, Dextrose 1.000gm, Ferrous sulphate 0.200 gm, Sodium chloride 5.000 gm, Sodium thiosulphate 0.300 gm Phenol red 0.024gm Agar 15.000 gm
Final pH ( at 25°C) 7.4±0.2
Method
- With an inoculating needle, pick the center of well-isolated colonies obtained from solid culture media. The medium is recommended for the identification of colonies picked off from plating media such as MacConkey Agar, Bismuth Sulphite Agar, or Desoxycholate Citrate Agar, etc.
- Stab the center of the medium into the deep of the tube to within 3-5mm from the bottom.
- Withdraw the inoculating needle and streak the surface of the slant.
- Loosen closure on the tube before incubating.
- Incubate aerobically at 35ºC. for 18-48 hours.
- Read tubes for acid production of the slant/butt, gas, and hydrogen sulfide reactions.
Expected Results
Carbohydrate Fermentation:
- Positive Test for Slant Reaction – Yellow (acid)
- Negative Test for Slant Reaction – Red (alkaline)
- Positive Test for Butt Reaction – Yellow (acid)
- Negative Test for Butt Reaction – Red (alkaline)
KIA Color Reactions:
- Red slant/ yellow butt – dextrose (+), lactose (-)
- Yellow slant/ yellow butt – dextrose (+), lactose (+)
- Red slant/ red butt – dextrose (-), lactose (-)
Hydrogen Sulfide Production:
- Positive Test – Black color throughout medium, a black ring at the juncture of the slant and butt, or a black precipitate in the butt
- Negative Test – No black color development
Gas Production:
- Positive Test – Bubbles in the medium, cracking and displacement of the medium, or separation of the medium from the side and bottom of the tube
- Negative Test – No bubbles and no separation or displacement of the medium.
Uses
- The test is recommended for the differential identification of gram-negative enteric bacilli from clinical and non clinical samples on the basis of the fermentation of dextrose, lactose and H2S production.
- It is used as a differentiation medium for typhoid, dysentery and allied bacilli.
- It differentiates Salmonella Typhi from other Salmonellae and also Salmonella Paratyphi A from Salmonella Scottmuelleri and Salmonella Enteritidis.
- Kligler Iron Agar test differentiates lactose fermenters from the nonfermenters.
Limitations
- It is recommended that biochemical, immunological, molecular, or mass spectrometry testing be performed on colonies from pure culture for complete identification.
- Failure to stab the butt invalidates this test.
- Certain species or strains may give delayed reactions or completely fail to ferment the carbohydrate in the stated manner.
- Results should be noted after 18-24 hours. Else it might result in erroneous results.
- Pure cultures are essential when inoculating Kligler Iron Agar. If inoculated with a mixed culture, irregular observations may occur.
- Hydrogen sulfide producing organisms may produce a black precipitate to such a degree that the reaction in the butt is completely masked. If hydrogen sulfide is produced, dextrose is fermented even if it is not observed.
- Hydrogen sulfide determinations using Kligler Iron Agar should be limited to members of Enterobacteriaceae.
References
- www.oxoid.com/UK/blue/prod_detail/prod_detail.asp?pr=CM0033&org=66&c=UK&lang=EN
- Downes F. P. and Ito K., (Ed.), 2001, Compendium of Methods for the Microbiological Examination of Foods, 4th Ed., American Public Health Association, Washington, D.C.
- himedialabs.com/TD/M078.pdf
- https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/KliglerIronAgarKIA.htm
- foodsafety.neogen.com/pdf/acumedia_pi/7140_pi.pdf
- https://assets.thermofisher.com/TFS-Assets/LSG/manuals/IFU453621.pdf
- https://mltgeeks.com/kligler-iron-agar-principlescomposition-and-slants-interpretation/
Similar Posts:
- The Triple Sugar Iron (TSI) Test – Principle, Procedure, Uses and Interpretation
- Lysine Iron Agar (LIA) Slants Test – Procedure, Uses and Interpretation
- Hydrogen Sulfide Test – Principle, Procedure, Uses and Interpretation
- Salmonella Shigella (SS) Agar- Composition, Principle, Uses, Preparation and Result Interpretation

How would you use kligler iron Agar ( KIA) to differentiate between shigella species and salmonella specie