- Rapid identification of gram-negative bacilli of the family Enterobacteriaceae isolated from clinical specimens has long posed a problem for the clinical laboratory.
- The group to which an organism belongs must be determined by a combination of biochemical tests, not by a single property.
- Various schema based on selected biochemical tests have been advocated to simplify the identification of Enterobacteriaceae.
- The Enterotube™ II is an example of a rapid, multi test system used in identification of unknown oxidase- negative, gram- negative, rod shaped bacteria of the family Enterobacteriaceae.
Objective
- To perform rapid differential identification of Gram-negative bacteria (Enterobacteriaceae) using the The Enterotube™ II.
Principle
The Enterotube™ II consists of a tube with a flat side and contains 12 compartments for different biochemical tests. It has prepared sterile multimedia tube for rapid differential identification of Gram-negative bacteria (Enterobacteriaceae). The self-contained, sterile, compartmented plastic tube contains twelve different conventional media plus a self-contained inoculating wire. Subsequent performance of 15 biochemical tests (glucose, gas production, lysine decarboxylase, ornithine decarboxylase, H2S, indole, adonitol, lactose, arabinose, sorbitol, Voges-Proskauer, dulcitol, phenylalanine deaminase, urea and citrate) from a single bacterial colony together with the interpretation guide allow identification of Enterobacteriaceae.
The Results Pad and Color Reaction Chart permit a rapid check of the positive reactions obtained with BBL Enterotube II. The checked positive numbers are totaled, and the composite number is then located in the Interpretation Guide to identify the organisms. Where two or more organisms are listed, the confirmatory tests required to further identify them are also given.
Media
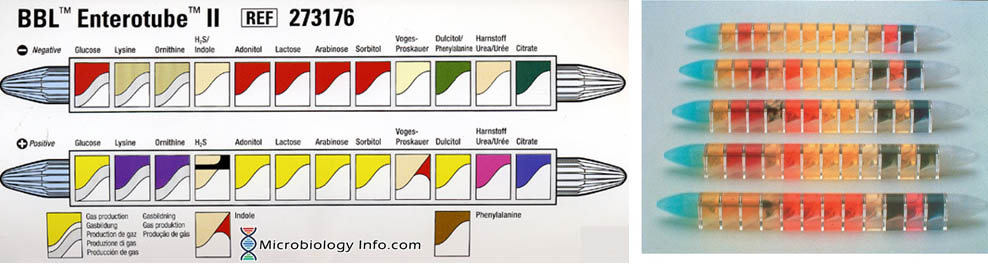
- Medium 1 (Glucose): Glucose (20.0 g/l) contained in an appropriate base medium, with cresol red as a pH indicator. The medium is covered with wax to provide anaerobic conditions and to allow detection of gas formation. Uninoculated: red.
- Medium 2 (Lysine): Lysine (10.0 g/l) contained in an appropriate base medium, with bromcresol purple as a pH indicator. The medium is covered with wax to provide anaerobic conditions. Uninoculated: yellow.
- Medium 3 (Ornithine): Ornithine (10.0 g/l) contained in an appropriate base medium, with bromcresol purple as a pH indicator. The medium is covered with wax to provide anaerobic conditions. Uninoculated: yellow.
- Medium 4 (H2S/Indole): Sodium thiosulfate (4.7 g/l) , ferric ammonium citrate (0.6 g/l) , and tryptophan (1.2 g/l) in an appropriate base medium. Uninoculated: Beige to light amber.
- Medium 5 (Adonitol): Adonitol (20.0 g/l) contained in an appropriate base medium, with cresol red as a pH indicator. Uninoculated: red.
- Medium 6 (Lactose): Lactose (20.0 g/l) contained in an appropriate base medium, with cresol red as a pH indicator. Uninoculated: red.
- Medium 7 (Arabinose): Arabinose (20.0 g/l) contained in an appropriate base medium, with cresol red as a pH indicator. Uninoculated: red.
- Medium 8 (Sorbitol): Sorbitol (20 g/l) contained in an appropriate base medium, with cresol red as a pH indicator. Uninoculated: red.
- Medium 9 (Voges-Proskauer): Glucose (20.0 g/l) contained in an appropriate base medium. Uninoculated: colorless to light amber.
- Medium 10 (Dulcitol/PA): Dulcitol (20.0 g/l) , phenylalanine (7.0 g/l), and ferric ammonium citrate (0.5 g/l) contained in an appropriate base medium, with bromthymol blue as a pH indicator. Uninoculated: green.
- Medium 11 (Urea): Urea (20.0 g/l) contained in an appropriate base medium, with phenol red as a pH indicator. Uninoculated: beige to light amber.
- Medium 12 (Citrate): Sodium citrate (2.0 g/l) contained in an appropriate base medium, with bromthymol blue as a pH indicator. Uninoculated: green.
Procedure
- Remove the caps from both ends to expose the inoculation wire. The wire is sterile and need not be flamed.
- Inoculate by touching the wire to a well isolated colony from a Petri plate. Inoculate the growth from MacConkey (MAC), Eosin Methylene Blue (EMB), Salmonella Shigella (SS) or Hektoen Enteric (HE) agars, or from nonselective blood agar media. The culture used should be at least 18 hours old and a pure culture of a Gram negative rod. Note: A visible amount of inoculum should be seen at the tip and the side of the wire. Avoid touching agar with wire.
- Inoculate BBL Enterotube II by first twisting wire, then withdrawing wire through all twelve compartments applying a turning motion
- Reinsert wire (without sterilizing) into BBL Enterotube II, using a turning motion through all 12 compartments, until the notch on the wire is aligned with the opening of the tube. The tip of the wire should be seen in the citrate compartment.
- Break wire at notch by bending. The portion of the wire remaining in the tube maintains anaerobic conditions necessary for true fermentation of glucose, production of as and decarboxylation of lysine and ornithine.
- With the broken off part of the wire, punch holes through the foil covering the air inlets of the last eight compartments (adonitol, lactose, arabinose, sorbitol, Voges-Proskauer, dulcitol/PA, urea and citrate) in order to support aerobic growth in these compartments.Replace both caps.
- Incubate at 35 to 37° C for 18 to 24 hours with BBL Enterotube II lying on its flat surface or in an upright position. Allow for air circulation between incubated tubes.
- Interpret and record all reactions with exception of indole and Voges-Proskauer. All other tests must be read before the indole and Voges-Proskauer tests are performed as the reagents added for these tests may alter the remainder of the BBL Enterotube II reactions.
Result Interpretation
- After 18 to 24 hours of incubation, interpret all reactions. With the exception of indole and VP, read the reactions in a sequential fashion by comparing the colors of the media in the tube after incubation with those given in the color scheme on the cover of the coding pad and with an uninoculated BBL Enterotube II which must be brought to room temperature first.
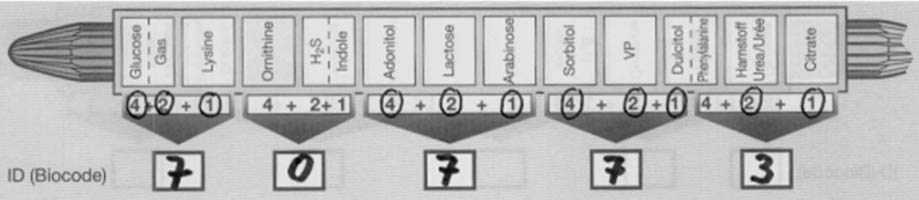
- Indicate each positive test result by circling the number appearing below the appropriate compartment on the Results Pad.
- Finally, perform the indole and VP tests. If positive, circle the appropriate numbers on the prepared sheet. Add circled numbers in the bracketed section and enter the sum in the space provided below the arrow.
- Locate the five digit number in the Interpretation Guide (codebook) and find the best answer(s) in the column entitled “ID Value“. Make sure to use the right database [(1) Oxidase negative nonfermenters, (2) Enterobacteriaceae – method without VP, and (3) Entero-bacteriaceae – method with VP].
Merits
- The Enterotube system provides a simple, reliable, and rapid method for the presumptive identification of Enterobacteriaceae.
- The major advantage of the Enterotube is that all tests are done simultaneously by inoculation from a single isolated colony.
- It reduces the number of inoculations and the equipment needed to perform a series of biochemical tests for the identification.
Limitations
- BBL Enterotube II is designed for the taxa provided. Taxa other than those explained are not intended for use in this system.
- BBL Enterotube II biocodes cannot be used to establish phenotypic identity between isolates from the same or different specimens.
- Biochemical results obtained with the BBL Enterotube II may differ from other methods and published material.
- Identification of Gram negative bacteria should be made with the consideration of additional characteristics such as source of specimen, history of the patient, colonial and microscopic morphology, serology and antimicrobial susceptibility patterns.
- Identification of rare isolates should be repeated or additional testing performed to verify the identification of such organisms.
- Some strains of organisms may exhibit atypical biochemical reactions due to unusual nutritional requirements or mutations and may be difficult to identify.
- Some organisms may require longer than 24 hours incubation for proper identification.
References
- Titsworth, E., Grunberg, E., Beskid, G., Cleeland, R., & Delorenzo, W. F. (1969). Efficiency of a multitest system (Enterotube) for rapid identification of Enterobacteriaceae. Applied microbiology, 18(2), 207-13.
- https://www.bd.com/europe/regulatory/Assets/IFU/HB/CE/ETUT/IA-273176.pdf
- https://www.wikidoc.org/index.php/Enterotube_II
- https://www.fishersci.com/shop/products/bd-bbl-enterotube-ii-tube-test-e-coli-testing-enterotube-ii-tube-test-12-media/rd43128
Similar Posts:
- Indole Test- Principle, Reagents, Procedure, Result Interpretation and Limitations
- Simmons Citrate Agar- Composition, Principle, Uses, Preparation and Result Interpretation
- Citrate Utilization Test- Principle, Media, Procedure and Result
- API (Analytical Profile Index) 20E Test – Procedure, Uses and Interpretation
