Most bacteria have the ability to ferment carbohydrates, particularly sugars. Among them, each bacteria can ferment only some of the sugars, while it cannot ferment the others. Thus, the sugars, which a bacteria can ferment and the sugars, which it cannot is the characteristic of the bacteria and thus an important criterion for its identification.
The Triple Sugar Iron (TSI) test is a microbiological test named for its ability to test a microorganism’s ability to ferment sugars and to produce hydrogen sulfide.
An agar slant of a special medium with multiple sugars constituting a pH-sensitive dye (phenol red), 1% lactose, 1% sucrose, 0.1% glucose, as well as sodium thiosulfate and ferrous sulfate or ferrous ammonium sulfate is used for carrying out the test.
All of these ingredients when mixed together and allowed solidification at an angle result in a agar test tube at a slanted angle. The slanted shape of this medium provides an array of surfaces that are either exposed to oxygen-containing air in varying degrees (an aerobic environment) or not exposed to air (an anaerobic environment) under which fermentation patterns of organisms are determined.
Objective
To determine the ability of an organism to ferment glucose, lactose, and sucrose, and their ability to produce hydrogen sulfide.
Principle
The triple sugar- iron agar test employing Triple Sugar Iron Agar is designed to differentiate among organisms based on the differences in carbohydrate fermentation patterns and hydrogen sulfide production. Carbohydrate fermentation is indicated by the production of gas and a change in the color of the pH indicator from red to yellow.
To facilitate the observation of carbohydrate utilization patterns, TSI Agar contains three fermentative sugars, lactose and sucrose in 1% concentrations and glucose in 0.1% concentration. Due to the building of acid during fermentation, the pH falls. The acid base indicator Phenol red is incorporated for detecting carbohydrate fermentation that is indicated by the change in color of the carbohydrate medium from orange red to yellow in the presence of acids. In case of oxidative decarboxylation of peptone, alkaline products are built and the pH rises. This is indicated by the change in color of the medium from orange red to deep red. Sodium thiosulfate and ferrous ammonium sulfate present in the medium detects the production of hydrogen sulfide and is indicated by the black color in the butt of the tube.
To facilitate the detection of organisms that only ferment glucose, the glucose concentration is one-tenth the concentration of lactose or sucrose. The meagre amount of acid production in the slant of the tube during glucose fermentation oxidizes rapidly, causing the medium to remain orange red or revert to an alkaline pH. In contrast, the acid reaction (yellow) is maintained in the butt of the tube since it is under lower oxygen tension.
After depletion of the limited glucose, organisms able to do so will begin to utilize the lactose or sucrose. To enhance the alkaline condition of the slant, free exchange of air must be permitted by closing the tube cap loosely.
Media:
TSI Agar
Enzymatic digest of casein (5 g), enzymatic digest of animal tissue (5 g), yeast enriched peptone (10 g), dextrose (1 g), lactose (10 g) sucrose (10 g), ferric ammonium citrate (0.2 g), NaCl (5 g), sodium thiosulfate (0.3 g), phenol red (0.025 g), agar (13.5 g), per 1000 mL, pH 7.3.
Method
- With a straight inoculation needle, touch the top of a well-isolated colony.
- Inoculate TSI by first stabbing through the center of the medium to the bottom of the tube and then streaking the surface of the agar slant.
- Leave the cap on loosely and incubate the tube at 35°-37°C in ambient air for 18 to 24 hours.
- Examine the reaction of medium.
Expected Results
- An alkaline/acid (red slant/yellow butt) reaction: It is indicative of dextrose fermentation only.
- An acid/acid (yellow slant/yellow butt) reaction: It indicates the fermentation of dextrose, lactose and/or sucrose.
- An alkaline/alkaline (red slant, red butt) reaction: Absence of carbohydrate fermentation results.
- Blackening of the medium: Occurs in the presence of H2
- Gas production: Bubbles or cracks in the agar indicate the production of gas ( formation of CO2and H2)
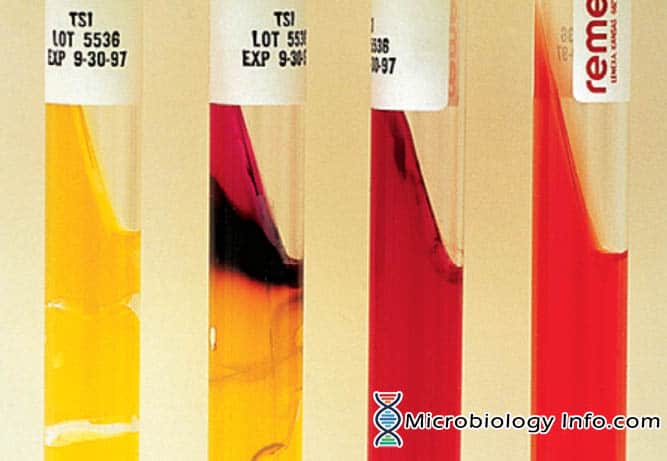
Triple sugar iron agar. A, Acid slant/acid butt with gas, no H2S (A/A). B, Alkaline slant/acid butt, no gas, H2S-positive (K/A H2S+). C, Alkaline slant/alkaline butt, no gas, no H2S (K/K). D, Uninoculated tube.
Uses
- The test is used primarily to differentiate members of the Enterobacteriaceae family from other gram-negative rods.
- It is also used in the differentiation among Enterobacteriaceae on the basis of their sugar fermentation patterns.
Limitations
- It is recommended that biochemical, immunological, molecular, or mass spectrometry testing be performed on colonies from pure culture for complete identification.
- It is important to stab the butt of the medium. Failure to stab the butt invalidates this test. The integrity of the agar must be maintained when stabbing. Caps must be loosened during this test or erroneous results will occur.
- TSI Agar must be read within the 18-24 hour stated incubation period. A false-positive reaction may be observed if read too early. A false-negative reaction may be observed if read later than 24 hours.
- An organism that produces hydrogen sulfide may mask acid production in the butt of the medium. However, hydrogen sulfide production requires an acid environment, thus the butt portion should be considered acid.
- TSI is not as sensitive in detecting hydrogen sulfide in comparison to other iron containing mediums, such as Sulfide Indole Motility (SIM) Medium.
- Certain species or strains may give delayed reactions or completely fail to ferment the carbohydrate in the stated manner.
References
- Tille P.M. 2014. Bailey and Scott’s diagnostic microbiology. Thirteen edition. Mosby, Inc., an affiliate of Elsevier Inc. 3251 Riverport Lane. St. Louis. Missouri 63043
- https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/TripleSugarIronTSIAgar.htm
- , et al. Manual of Clinical Microbiology, American Society for Microbiology, Washington, D.C.
- https://vlab.amrita.edu/?sub=3&brch=76&sim=216&cnt=1

Clear and well understood
I so much love the procedure. It is well detailed