Properties of Immunoglobulin E (IgE)
- Immunoglobulin E (IgE) is defined by the presence of the epsilon heavy chain in the structure.
- IgE exists as a monomer and is the least abundant antibody isotype in plasma, present at levels (about 100 ng/mL), approximately 300-fold lower than that of IgG in a circulation and accounts for 0.002% of total immunoglobulin.
- IgE has a very short half-life which is less than 1 day. The reason is because some proportion of the circulating amount is continually removed and destroyed in endosomes.
- Despite the low concentrations in the circulation, IgE is extremely biologically active. This is because although the concentration of IgE in the circulation is very low, IgE antibodies bind to high-affinity receptors present on the surface of mast cells and basophils, so that these cells may be highly sensitive to allergens.
- FceRI is a high-affinity receptor specific for IgE present at a high density on tissue-resident mast cells and basophils.
- Because of this high-affinity interaction, almost all IgE produced by B cells is bound to mast cells or basophils, which also explains the low concentration found in circulation.
- Cross-linking of the FceRI-bound IgE leads to cellular activation, resulting in immediate release of preformed granular components such as histamine and tryptase and subsequent production of lipid mediators (prostaglandins and leukotrienes) and cytokines (IL-4 and IL-5).
- In addition, the expression of the high-affinity receptors is upregulated during allergen-induced rhinitis in humans, probably by IgE itself. This concludes that the concentration of circulating IgE does not reflect its true activity.
- Under some conditions, IgE reaches pathological levels. Clinical diseases associated with unusually high elevation of serum IgE concentrations (>1,000 IU/mL) include atopic diseases (asthma, allergic rhinitis, atopic dermatitis, urticaria), parasitic diseases, cutaneous diseases, neoplastic diseases, and immune deficiencies.
Structure of Immunoglobulin E (IgE)
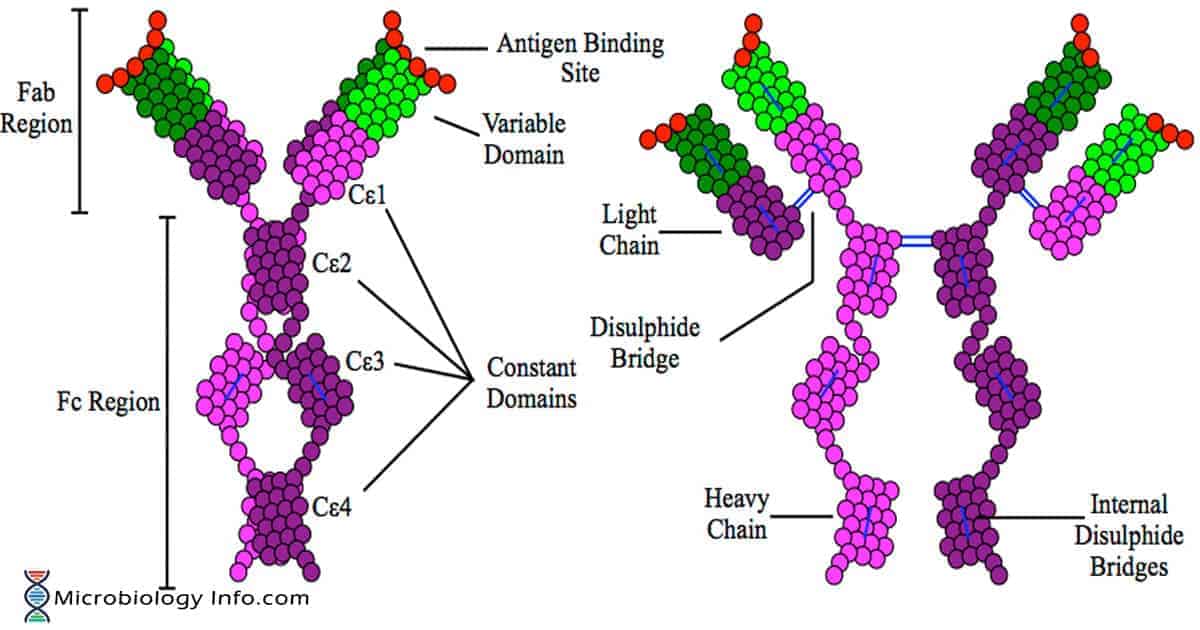
- IgE is a glycoprotein of 190KDa molecular weight produced as a monomeric antibody comprising of 2 epsilon(e)-heavy and 2 light chains (? or ?) linked by numerous intrachain disulfide bonds.
- IgE is found similar to IgG in structure, but IgE comprises two unique features: The epsilon (e) heavy chain which consists of high (12%) carbohydrate content and an additional constant region (CH4).
- The structure has two identical antigen binding areas consisting of both light chain (23KDa) and heavy chains (70KDa) and a valency of 2.
- In addition to the disulfide bonds linking the chains together, there are intrachain disulfide links that divide each chain into areas called as domains.
- The light chains have two domains, one constant and one variable.
- The heavy chains have five domains, one variable and four constant -region domains.
- It is unique in having the additional constant region (CH4).
- Heavy and light chains have variable regions on their most N terminal ends.
- Variable (V) sequences at the N-termini of the heavy (VH) and light (VL) chains create unique antigen-specific binding sites.
- The C-terminal regions of the e-heavy chains are made up of 4 Ce domains, each encoded by one of the Ce1 to Ce4 exons located near the 3′ end of the heavy chain locus (IgH).
- The IgE Ce2–4 Fc domains confer its isotype-specific functions, including binding to its receptors, FceRI and CD23.
- The unique CH4 region restricts IgE binding to high-affinity receptors (Fce-RI) on the surface of basophils and mast cells, which contain preformed granules of heparin and histamine.
- A hinge region is absent in the structure of IgE.
- Unlike Fc? and Fcµ, Fce does not activate complement.
- IgE antibodies are more heavily glycosylated than other immunoglobulin isotypes with 7N-linked glycosylation consensus sequences on each e-heavy chain, one of which is required for IgE binding to its high-affinity receptor, FceRI.
- As a result of this heavy glycosylation, IgE antibodies have an affinity for galectins, lectin-type proteins that can interact with both free and cell-bound IgE.
Functions of Immunoglobulin E (IgE)
- It primarily provides protection against helminth parasites but can also respond to foreign substances even in small amounts and is designated as a “gatekeeper.”
- Allergic reactions are predominantly associated with IgE. The function of IgE antibody as mediators in allergic reactions of Type I is explained by their ability to interact both with antigen and with receptor molecules on the membrane of blood basophils and tissue mast cells in the human body.
- Antigen-induced cross-linkage of receptor-bound IgE initiates a process that results in the release of histamine and heparin, which increase vascular permeability and promote contraction of smooth muscle.
- IgE-mediated mechanisms are conventionally known to facilitate degranulation of mast cells and basophils and promote TH2 immunity, mechanisms that are not only directed to mounting an appropriate defense against parasitic worms, noxious substances, toxins, venoms, and environmental irritants but that also trigger vigorous allergic reactions in patients having allergies.
- IgE has an essential role in type I hypersensitivity, which manifests in assorted allergic diseases, such as food allergies, allergic asthma, most types of sinusitis, allergic rhinitis, and specific types of atopic dermatitis and chronic urticaria.
- In certain cases or conditions high IgE levels indicate that the body is overreacting to allergens leading to an allergic reaction. It can be considered as a sign that the body is fighting off an infection from a parasite or with some immune system conditions.
- In addition to a role in allergic reactions, IgE plays a significant role in the immune response to parasites such as Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. IgE is utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum.
- Release of histamine induces muscular contractions in the intestine, which contribute to the expulsion of parasites.
- IgE also appears to play a role in resisting ticks of the Ixodid family, which carry among other diseases the organisms for Lyme disease and ehrlichiosis, however, the exact workings of IgE remain unclear.
- IgE also plays a significant role in responses to allergens, such as: anaphylactic drugs, bee stings, and antigen preparations used in desensitization immunotherapy.
References
- Unal, D., Gelincik, A., Elitok, A., Demir, S., Olgac, M., Coskun, R., Kocaaga, M., Colakoglu, B., Buyukozturk, S. (2017). Impact of high serum Immunoglobulin E levels on the risk of atherosclerosis in humans. Asia Pacific allergy, 7(2), 74-81.
- Brown, W. R., Borthistle, B. K., & Chen, S. T. (1975). Immunoglobulin E (IgE) and IgE-containing cells in human gastrointestinal fluids and tissues. Clinical and experimental immunology, 20(2), 227-37.
- Bennich H., Johansson S.G.O., von Bahr-Lindström H., Karlsson T. (1976) Function and Structure of Immunoglobulin E (IgE). In: Johansson S.G.O., Strandberg K., Uvnäs B. (eds) Molecular and Biological Aspects of the Acute Allergic Reaction. Springer, Boston, MA.
- Fifty years later: Emerging functions of IgE antibodies in host defense, immune regulation, and allergic diseases. (2016). The Journal of allergy and clinical immunology, 137(6), 1631-1645.
- Abbas A.K and Lichtman A.H. Cellular and molecular immunology. Fifth edition. Page no.35-43.
- Owen, J. A., Punt, J., & Stranford, S. A and Jones, P.P (2013). Kuby Immunology (7 ed.). New York: W.H. Freeman and Company.
- Delves, P.J., Martin, S.J., Burton, D.R. and Roitt, I.M. (2011). Roitt’s Essentail Immunology. 12th edition. A John Wiley and Son’s, Ltd, Publication.
- Cruse, J.M. and Lewis, R.E. (2010). Atlas of Immunology. Third edition. CRC Press. Taylor and Francis Group, 6000 Broken Sound Parkway NW, Suite 300, Boca Raton, FL 33487-2742.
Image by: SariSabban / CC BY-SA (https://creativecommons.org/licenses/by-sa/3.0)

Wonderful topic explained in simple and lucid order. Hats off. VTRAJ