Carbohydrates are organic molecules that contain carbon, hydrogen and oxygen in the ratio (CH2O)n. Organisms use carbohydrate differently depending upon their enzyme complement.
The pattern of fermentation is characteristics of certain species, genera or groups of organisms and for this reason this property has been extensively used as method for biochemical differentiation of microbes.
Objective
To detect the oxidation or fermentation of carbohydrates by bacteria.
Principle
Whether an organism is oxidative or fermentative can be determined by using Hugh and Leifson’s medium, commonly called as OF medium which contain tryptone and bromothymol blue (an indicator). One of the sugars, such as glucose, xylose, mannitol, lactose, sucrose, and maltose is added to the medium which serves as the fermentable carbohydrate.
An organism is inoculated to two tubes of each OF Medium. Once inoculated, one tube is overlaid with mineral oil or melted paraffin producing an anaerobic environment. The other tube is left open to the air. Growth of microorganisms in this medium is either by utilizing the tryptone which results in an alkaline reaction (dark blue color) or by utilizing glucose, which results in the production of acid (turning bromothymol blue to yellow).
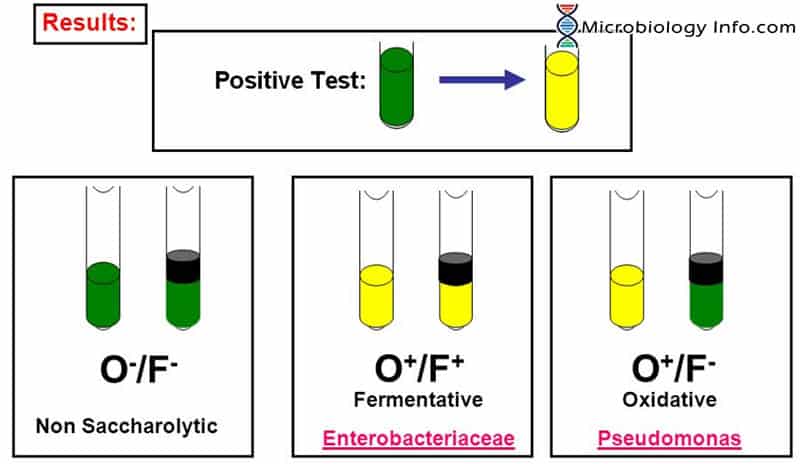
Oxidative utilization of the carbohydrate will result in acid production (yellow) in the open tube only. Fermentative utilization of the carbohydrate will result in acid production (yellow) in both the open and closed tubes. Acidic changes in the overlaid tubes are considered to be a result of true fermentation, while acidic development in the open tubes are due to oxidative utilization of the carbohydrate present. Asaccharolytic organisms will not produce acid in either tube.
Media:
Hugh and Leifson’s medium: Peptone 2.0gm/L, Sodium chloride 5.0gm/L, Dipotassium phosphate 0.30gm/L, Glucose (Dextrose) 10.0gm/L, Bromothymol blue 0.030gm/L, Agar 3.0gm/L, Final pH ( at 25°C) 7.1±0.2
Method
- Obtain pure, isolated colonies from an 18-24 hour culture.
- For each test organism, inoculate tubes in duplicate. Inoculate by stabbing the agar to approximately 1/4 inch from the bottom.
- Apply sterile mineral oil, sterile melted paraffin, or sterile melted petrolatum to one of each duplicate tubes. Tighten the cap of the overlaid tube, and loosen the cap of the non-overlaid tube.
- Incubate both tubes aerobically at 35ºC. for up to 14 days.
- Examine tubes daily for color change.
Expected Results
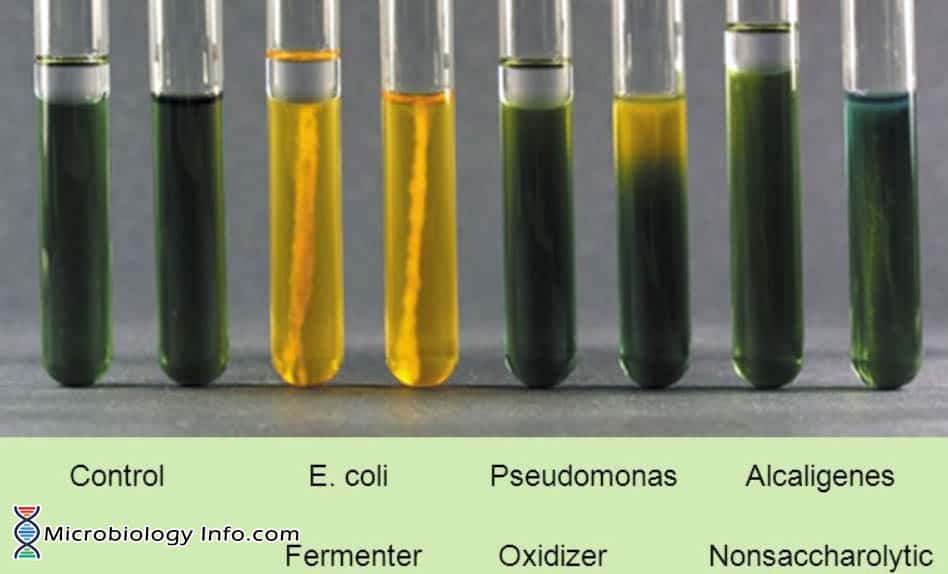
- Positive: A positive carbohydrate utilization test is indicated by the development of a yellow color in the medium.
- Oxidative:Development of a yellow coloration in the open tube only.
- Fermentative: Development of a yellow coloration in both open and closed tubes.
- Negative: A negative carbohydrate utilization test is indicated by the absence of a yellow color (media remains green or turns blue).
- Non-oxidizer/Non-fermenter
Uses
- It aids in the identification of gram-negative bacteria on the basis of their ability to oxidize or ferment a specific carbohydrate.
- It is used to determine whether an organism uses carbohydrate substrates to produce acid byproducts.
- Non fermentative bacteria are routinely tested for their ability to produce acid from six carbohydrates (glucose, xylose, mannitol, lactose, sucrose, and maltose).
Limitations
- It is recommended that biochemical, immunological, molecular, or mass spectrometry testing be performed on colonies from pure culture for complete identification.
- Slow-growing organisms may not produce results for several days.
- Some microorganisms do not grow in OF Medium. It may be necessary to use another basal medium containing dextrose to confirm the negative reaction.
- Some mineral oils are acidic and may result in erroneous results.
References
- Cappuccino J.G. and Sherman N. 2008. Microbiology: A Laboratory Manual, 8th ed. Pearson Benjamin Cummings, San Francisco, CA, USA
- Murray, P.R., E.J. Baron, J.H. Jorgensen, M.L. Landry, and M.A. Pfaller. 2007. Manual of Clinical Microbiology. 9th ed. ASM Press, Washington, D.C.
- https://catalog.hardydiagnostics.com/cp_prod/content/hugo/ofbasalmedium.htm
- asmscience.org/content/education/protocol/protocol.3151